A sample of unknown metal is placed in a graduated cylinder containing water. The mass of the
Question:
A sample of unknown metal is placed in a graduated cylinder containing water. The mass of the sample is 37.5 g, and the water levels before and after adding the sample to the cylinder are as shown in the figure. Which metal in the following list is most likely the sample? (d is the density of the metal.)
(a). Mg, d = 1.74 g/cm3
(b). Fe, d = 7.87 g/cm3
(c). Ag, d = 10.5 g/cm3
(d). Al, d = 2.70 g/cm3
(e). Cu, d = 8.96 g/cm3
(f). Pb, d = 11.3 g/cm3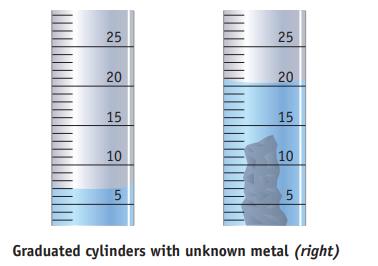
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





