The following rate constants were obtained in an experiment in which the decomposition of gaseous N 2
Question:
The following rate constants were obtained in an experiment in which the decomposition of gaseous N2O5 was studied as a function of temperature. The products were NO2 and NO3.
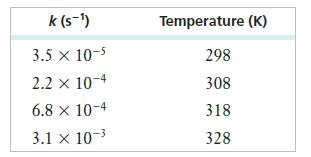
Determine Ea for this reaction in kJ/mol.
Transcribed Image Text:
k (S-¹) 3.5 x 10-5 2.2 x 10-4 6.8 x 10-4 3.1 x 10-³ Temperature (K) 298 308 318 328
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
E ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
As the air temperature drops, river water becomes supercooled and ice crystals form. Such ice can significantly affect the hydraulics of a river. The article "Laboratory Study of Anchor Ice Growth"...
-
The reaction C 2 H 5 I + OH - C 2 H 5 OH + I - was studied in an ethanol (C 2 H 5 OH) solution, and the following rate constants were obtained: 15.83 C, k = 5.03 x 10 -5 ; 32.02 C, 3.68 x 10 -4 ;...
-
For the reaction I (aq) + OCl (aq) OI (aq) + Cl (aq) occurring in aqueous solution, the following mechanism has been proposed: a. Derive the rate law expression for this reaction based on this...
-
A house girl added 20g of sodium chloride (NaCl) to 80g of water (atomic masses are Na=23amu, Cl=35.5amu). Calculate a)Percent(w/w) of NaCl b)Mole fraction of NaCl
-
On January 1, the company had 150,000 common shares outstanding. During the year, the following events occurred: March 1: 2-for-1 stock split June 1: Issued 45,000 additional shares September 1: 20%...
-
A nonprofit government corporation is considering two alternatives for generating | power: Alternative A. Build a coal-powered generating [facility at a cost of $20,000,000. Annual power sales are...
-
Four independent situations are given below. Assume partners of the existing partnership are Maria Heath and Lisa Curtis. The new partner is Wade Torres. For each situation, prepare the appropriate...
-
Phelan Corporation and Keevin Corporation, two companies of roughly the same size, are both involved in the manufacture of shoe-tracing devices. Each company depreciates its plant assets using the...
-
Debate: Is international law useful or a fallacy ultimately doomed by politics? Arguments in support of International Law being useful, and against this, instead arguing that it is a fallacy doomed...
-
The table below presents measured rate constants for the reaction of NO with ozone at three temperatures. From these data, determine the activation energy of the reaction in kJ/mol. (Assume the...
-
Use the kinetic-molecular theory to explain why an increase in temperature increases reaction rate.
-
Harley-Davidson, lnc., is a leading motorcycle manufacturer in the United States. The company manufactures and sells a number of different types of motorcycles, a complete line of motorcycle parts,...
-
Write a thesis on income equality in India, write something about Income Equality in India, and compare it with Income Equality in Canada.
-
For your final project, you'll need access to the case file from Harvar and the link for purchase is https://hbsp.harvard.edu/import/1046501Links to an external site. (Links to an external site.)(...
-
Yaster Gadgets manufactures and sells a smartphones per week. The weekly price-demand and cost equations are, respectively, p = 484 -0.45 x and C(x) = 20,081 +20x. Suppose Yaster Gadgets wants to...
-
1. If a rock fell from a very high cliff, how far would it drop in 5.0 seconds? Ignore air resistance 2. A stunt car travelling horizontally at 13.0 m/s drives off the edge of a cliff 5.6 m high. How...
-
Before completing this activity, be sure to review and refer to the following resources to become familiar with a Balanced Scorecard: 6.8 Your Personal Balanced Scorecard - Principles of Management...
-
The following information pertains to Hagen Metal Works ending inventory for the current year: Required a. Determine the value of the ending inventory using the lower-of-cost-or-market rule applied...
-
4. Jobe dy -Y 2 et by
-
Oxygen sensing is important in biological studies of many systems. The variation in oxygen content of sapwood trees was measured by del Hierro and coworkers [ J. Experimental Biology 53 (2002): 559]...
-
The pyrene/coumarin FRET pair (r 0 = 39 ) is used to study the fluctuations in enzyme structure during the course of a reaction. Computational studies suggest that the pair will be separated by 35 ...
-
In a FRET experiment designed to monitor conformational changes in T4 lysozyme, the fluorescence intensity fluctuates between 5000 and 10,000 counts per second. Assuming that 7500 counts represent a...
-
Keiko is buying a used car. Payments will be $ 7 8 . 7 5 every week for 2 years, with the first payment at the end of 1 3 weeks. The interest rate is 7 . 0 7 5 % compounded monthly. What is the...
-
Sosa Company has $39 per unit in variable costs and $1,900,000 per year in fixed costs. Demand is estimated to be 138,000 units annually. What is the price if a markup of 35% on total cost is used to...
-
Do you think employers have a right to check into applicants' backgrounds? What about if that check includes social media, blogs, pictures, or writings that can be found on the internet? Mountain Ski...

Study smarter with the SolutionInn App


