Calculate the enthalpy change for using the thermochemical equations C2H,(g) + HO(0) CHOH(0) = ?
Question:
Calculate the enthalpy change for![]()
using the thermochemical equations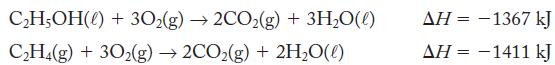
Transcribed Image Text:
C2H,(g) + HO(0) → CHOH(0) ΔΗ = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

David Ngaruiya
i am a smart worker who concentrates on the content according to my clients' specifications and requirements.
4.50+
7+ Reviews
19+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Th e chemical industry converts hydrocarbons of low molecular mass to larger and more useful compounds. Calculate the change in enthalpy for the synthesis of cyclohexane (C 6 H 12 ), a compound used...
-
Hydrogenation of hydrocarbons is an important reaction in the chemical industry. A simple example is the hydrogenation of ethylene to form ethane. Calculate the enthalpy change for Use the following...
-
The chapter introduction introduced the following reaction as one chemical reaction used to launch the space shuttle. Calculate the mass of aluminum required to generate 60,500 kJ energy (enough to...
-
Dylan Flaherty, marketing clerk for TipTop Marketing Agency, recorded the following information for last year: He would like to be able to estimate customer service costs using the number of...
-
Shen and Smith [Ind. Eng. Chem. Fundam., 7, 100-105 (1968)] measured equilibrium-adsorption isotherms at four different temperatures for pure benzene vapor on silica gel, having the following...
-
I owe $10,000 on one credit card that charges 18 percent annual interest and $5,000 on another credit card that charges 12 percent annual interest. Interest for the month is based on the months...
-
Hi Tech Corporation provides customers with access to the latest advancements in technology and software. Hi Tech replaces employee workstation computers every three years. Printers are replaced...
-
John Doe, a fraud examiner, has been hired by ABC Corporation to investigate a shortage of cash, which management thinks is being caused by fraudulent behavior. John Doe could spend his time and...
-
19. Find the tension T for the system shown in figure :- T T T 1 kg 2 kg 3 kg (1) IgN (2) 2 gN (3) 5 gN (4) 6 gN 20. A ball of mass 0.5 kg moving with a velocity of 2 m/sec strikes a wall normally...
-
Name the fallacy in each statement below and explain why it is fallacious. 1. The best restaurant in New York city is either buddy's bistro or clyde's emporium. 2. Everyone is going to buddy's...
-
Differentiate between kinetic energy and potential energy.
-
A 50.0 g-sample of acid takes 46.4 mL of 0.500 M NaOH solution to neutralize it. Assume the same amount of heat is given off as in Example 5.6. (a) Calculate the enthalpy change for the...
-
Explain relationships between economic strikes and unfair labor practice strikes.
-
The defendant was convicted of unlawfully selling narcotic drugs. The Criminal Court, Cook County, Richard B. Austin, J., rendered a judgment, and the defendant brought error. The Supreme Court,...
-
The United States has a dual court system that separates federal and state courts. America is characterized by cooperative federalism, meaning some of the lines between federal and state power are...
-
A gas has the equation of state \[\frac{p v_{m}}{\Re T}=1+\mathrm{A} p\left(T^{3}-9.75 T_{\mathrm{c}} T^{2}+9 T_{\mathrm{c}}^{2} T ight)+\mathrm{B} p^{2} T\] where \(\mathrm{A}\) and \(\mathrm{B}\)...
-
We are a global, science-led biopharmaceutical business. Return to shareholders Revenue from the sale of our medicines generates cash flow, which helps us fund business investment. It also enables us...
-
The police found marijuana and growing equipment when they searched a mans house. He was prosecuted criminally, but the federal government also sought civil forfeiture of his house, pursuant to a...
-
A start-up company is moving into its first offices and needs desks, chairs, filing cabinets, and other furniture. It can buy the furniture for $25,000 or rent it for $1,500 per month. The founders...
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
Draw the enol of each of the following compounds, and identify whether the enol exhibits a significant presence at equilibrium. Explain. (a) (b) (c) (d)
-
Ethyl acetoacetate has three enol isomers. Draw all three.
-
Draw the enolate that is formed when each of the following compounds is treated with LDA. (a) (b) (c) (d)
-
bugoze limited bought for cash 25 acres of land at a cost of $215,000 during the year. About two months later the land was sold to a developer for $ 500,000 consisting of a $ 220,000 down payment in...
-
A) What is the above organisational structure? Justify giving 2 characteristics.
-
1. What is an Ad Idea? How does it impact your creative? 2. Explain an advertisement that you love, or find online. How does the creative and copy power the underlying message for that brand/product?...

Study smarter with the SolutionInn App


