Calculate the enthalpy change observed in the combustion reaction of 1.00 g ethane, using the thermochemical equation.
Question:
Calculate the enthalpy change observed in the combustion reaction of 1.00 g ethane, using the thermochemical equation.
Thermochemical Equation![]()
Strategy
We will use the same approach as in previous stoichiometry calculations. Convert the mass of ethane to moles; then use stoichiometric relations in the thermochemical equation to calculate ΔH.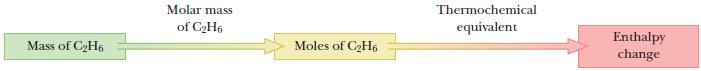
Transcribed Image Text:
2C₂H6(g) + 702₂(g) → 4CO₂(g) + 6H₂O(l) ΔΗ = -3120 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The equation given earlier in this section is First we convert the mass o...View the full answer

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Circle the functional groups that are eliminated in the formation of a peptide bond between the amino acids shown below. Draw the structure of the dipeptide. R1 H R2 H C-C-N H C-C-N H
-
The chapter introduction introduced the following reaction as one chemical reaction used to launch the space shuttle. Calculate the mass of aluminum required to generate 60,500 kJ energy (enough to...
-
Th e chemical industry converts hydrocarbons of low molecular mass to larger and more useful compounds. Calculate the change in enthalpy for the synthesis of cyclohexane (C 6 H 12 ), a compound used...
-
Joseph Thompson is president and sole shareholder of Jay Corporation. In December 2019, Joe asks your advice regarding a charitable contribution he plans to have the corporation make to the...
-
A reverse-osmosis plant is used to treat 30,000,000 gal/day of seawater at 20C containing 3.5 wt% dissolved solids to produce 10,000,000 gal/day of potable water with 500 ppm of dissolved solids, and...
-
The atmospheric pressure decreases with increasing altitude. At sea level, the average air pressure is one atmosphere (1.033227 kilograms per square centimeter). The table shows the pressures p (in...
-
List and describe the legal elements that comprise the unintentional tort of negligence. Provide an example of a negligence situation involving a personal injury. Provide another example of a...
-
Raquel is an employee of Jones Company and owns a 30% interest in the company. Her salary is $44,000. She also receives a $10,000 cash distribution from Jones. During the current year, Joness...
-
Fluorine Limited (FL), a manufacturer of ships, has entered into the following contracts during the year ended 31 December 2022: (i) On 1 January 2022, FL entered into a contract with Alpha Limited...
-
1. Which aspect of the French revolution most disturbed commentators? 2. How would you align each of these writers on a spectrum running from extreme right to extreme left in politics? 3. How would...
-
Why must the physical states of all reactants and products be specified in a thermochemical equation?
-
Use Equation 5.2 to calculate the enthalpy change when 5.00 g O 2 is consumed by reaction with N 2 , forming NO. Equation 5.2 N(g) + O(g) - 2NO(g) = +181.8 kJ
-
In a 2018 study reported in The Lancet, a randomized, double-blind controlled experiment was conducted to determine the effect of the drug upadacitinib on patients with active rheumatoid arthritis....
-
The financial crisis that hit the United States first and then the world economy starting in fall 2007 meant that the future prospects of many firms looked gloomy at best for some time. Comment on...
-
LinkedIn (www.linkedin.com) is the largest and best-known social network for professionals. Many of you are probably already familiar with it. 1. Do you have a profile? If not, you might want to...
-
Draw up a table listing the advantages and disadvantages of e-mail marketing compared with more traditional approaches to direct marketing. In what kind of situations do you think e-mail marketing...
-
Open a new spreadsheet. In Cell A1, type Spending Categories. In Cell A3, type Salaries; in cell A4, type Fringe Benefits; in Cell A5, type OTPS; in Cell A6, type Total. a. Adjust the column width,...
-
You are the owner of a mid-sized insurance company. You have 25 agents who travel throughout the country, making presentations to small groups of 1020 people. You want to create a template that all...
-
In January 2009, a one-year call on the stock of Amazon.com, with an exercise price of $45.00, sold for $19.55. The stock price was $55. The risk-free interest rate was 2.5%. How much would you be...
-
Nate prepares slides for his microscope. In 1 day he prepared 12 different slides. Which equation best represents y, the total number of slides Nate prepares in x days if he continues at this rate? A...
-
Rank this group of compounds in order of increasing boiling point. -NH2 -N-
-
Identify whether each of the following compounds is expected to be water soluble: (a) (b) (c) -NH2 -NH2
-
For each of the following pairs of compounds, identify which compound is the stronger base: (a) (b) (c) (d) N- N- N- N-
-
Cash Flow for ABC Hotel July August September Opening Balance $ 85,000 $ 88,450 ? Cash Incoming Sales $ 67,000 $ 64,399 $ 62,500 Asset Sales $ 1,200 $ - $ - Debtor Receipts $ 2,500 $ 2,800 $ 1,300...
-
Describe at a high level the various cost categories and expenditures.Describe in detail the significant items in the budget, including how these expenses and revenues were determined and why they...
-
What are the metabolic adaptations underlying cancer metabolism, including the Warburg effect, glutamine addiction, and alterations in nucleotide metabolism, and how do these metabolic rewiring...

Study smarter with the SolutionInn App


