Calculate the value of the solubility product constant for Cd(OH) 2 from the half-cell potentials.
Question:
Calculate the value of the solubility product constant for Cd(OH)2 from the half-cell potentials.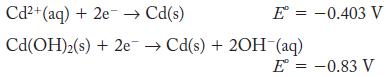
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The question presents us with halfcell potentials for two reactions involving cadmium To calculate t...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix 2B and the fact that, for the half-reaction F 2 (g) + 2 H + (aq) + 2 e 2 HF(aq), E = 13.03 V, to calculate the value of K a for HF. 2B STANDARD POTENTIALS AT 25 C...
-
As in Example 6L.1, you are planning to use a Daniell cell to power a model electric car. However, you find that you do not have standard solutions available. You have only dilute solutions, and you...
-
Calculate the solubility product constant for copper(II) iodate, Cu(IO3)2. The solubility of copper(II) iodate in water is 0.13 g/100 mL.
-
What does 'buying local' mean to you? How do you determine what 'local' means? Are there limits to buying local here in PEI? Is buying local a priority for you when making purchases? How do you...
-
Determine F12 and F21 for the following configurations using the reciprocity theorem and other basic shape factor relations. Do not use tables or charts. (a) Long duct (b) Small sphere of area A1...
-
Compute the fraction of the bandwidth that is wasted on overhead (headers and retransmissions) for protocol 6 on a heavily-loaded 50-kbps satellite channel with data frames consisting of 40 header...
-
True or False: Only electric motors on variable-frequency drives (VFDs) have electric discharge machining (EDM) damage.
-
Martin Acampora purchased a shotgun at a garage sale years ago, never used the weapon, and did not know of any defects in it. His 31-year-old son Marty borrowed the shotgun to go duck hunting. As...
-
The following table contains financial information for Dillon Incorporated before closing entries: Cash Supplies $ 12,000 4,500 Prepaid Rent 2,000 Salaries Expense 4,500 Equipment 65,000 Service...
-
Calculate the value of the solubility product constant for PbSO 4 from the half-cell potentials.
-
Using the voltaic cell in Exercise 18.64 , a voltage of 0.425 V was measured when the cell was placed in a solution of unknown ion concentrations. What is the ratio [Cd 2+ ]/[Ag + ] 2 in this...
-
When property taxes become delinquent, governmental entities often accrue interest and penalties on the delinquent taxes or choose to sell the rights to collect such taxes, interest, and penalties to...
-
What are the main changes to channel structures that are facilitated through the Internet?
-
Trading online increasingly involves developing multichannel strategies. Give three examples of potential channel conflicts that might arise from using the Internet. Illustrate your answer with...
-
Setting long-term strategic objectives for a website is unrealistic since the rate of change in the marketplace is so rapid. Discuss.
-
Summarise how each of the micro-environment factors may directly drive the content and services provided by a website.
-
What is the purpose of a digital marketing audit? What should it involve?
-
Amazon.com, Inc. like all other businessesadjusts accounts prior to year end to get correct amounts for the financial statements. Examine Amazon.com, Inc.s Consolidated Balance Sheets in Appendix A,...
-
(a) As Section 17.3 discusses, high-frequency sound waves exhibit less diffraction than low-frequency sound waves do. However, even high-frequency sound waves exhibit much more diffraction under...
-
A barge is 60 ft long, 20 ft wide, and 8 ft deep. When empty, it weighs 210 000 lb, and its center of gravity is 1.5 ft above the bottom. Is it stable when floating in water?
-
If the barge in Problem 5.57 is loaded with 240 000 lb of loose coal having an average density of 45 lb/ft 3 , how much of the barge would be below the water? Is it stable? In Problem A barge is 60...
-
A piece of cork having a specific weight of 2.36 kN/m 3 is shaped as shown in Fig. 5.32. (a) To what depth will it sink in turpentine (sg = 0.87) if placed in the orientation shown? (b) Is it stable...
-
Identify two marketing actions to improve the LTV of your chosen segment. Explain how these actions would improve this segment's LTV. The segment I choose is Young Market Segment.
-
Fuzzy Monkey Technologies, Inc., purchased as a long - term investment $ 2 5 0 million of 8 % bonds, dated January 1 , on January 1 , 2 0 2 1 . Management intends to have the investment available for...
-
Low Country Goods has four employees and pays them on an hourly basis. During the week beginning June 2 4 and ending June 3 0 , these employees worked the hours shown below. Information about hourly...

Study smarter with the SolutionInn App


