Calculate the value of the solubility product constant for PbSO 4 from the half-cell potentials.
Question:
Calculate the value of the solubility product constant for PbSO4 from the half-cell potentials.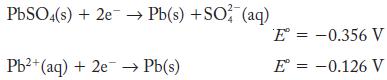
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
E 0073 V ...View the full answer

Answered By

Bree Normandin
Success in writing necessitates a commitment to grammatical excellence, a profound knack to pursue information, and a staunch adherence to deadlines, and the requirements of the individual publication. My background comprises writing research projects, research meta-analyses, literature reviews, white paper reports, multimedia projects, reports for peer-reviewed journals, among others. I work efficiently, with ease and deliver high-quality outputs within the stipulated deadline. I am proficient in APA, MLA, and Harvard referencing styles. I have good taste in writing and reading. I understand that this is a long standing and coupled with excellent research skills, analysis, well-articulated expressions, teamwork, availability all summed up by patience and passion. I put primacy on client satisfaction to gain loyalty, and trust for future projects. As a detail-oriented researcher with extensive experience surpassing eight years crafting high-quality custom written essays and numerous academic publications, I am confident that I could considerably exceed your expectations for the role of a freelance academic writer.
5.00+
7+ Reviews
21+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculate the solubility product constant for copper(II) iodate, Cu(IO3)2. The solubility of copper(II) iodate in water is 0.13 g/100 mL.
-
Use the data in Appendix 2B and the fact that, for the half-reaction F 2 (g) + 2 H + (aq) + 2 e 2 HF(aq), E = 13.03 V, to calculate the value of K a for HF. 2B STANDARD POTENTIALS AT 25 C...
-
(a) Use data from Appendix 2B to calculate the solubility product of Hg 2 Cl 2 . (b) Compare this number with the value listed in Table 6I.1 and comment on any difference. TABLE 61.1 Solubility...
-
Read the following facts and then choose the correct option below: X acts as an interpreter in a transaction where Y wants to buy stolen goods from Z. X's conduct is considered to be that of a/an: a....
-
Consider the following grooves, each of width W, that have been machined from a solid block of material. (a) For each case obtain an expression for the view factor of the groove with respect to the...
-
In protocol 6, MAX_SEQ = 2n - 1. While this condition is obviously desirable to make efficient use of header bits, we have not demonstrated that it is essential. Does the protocol work correctly for...
-
True or False: The damage shown in the image below, due to EDM, takes several years to occur.
-
Laminar flow in a narrow slit (see Fig. 2B.3).? (a) A Newtonian fluid is in laminar flow in a narrow slit formed by two parallel walls a distance 2B apart. It is understood that B p + pgh = p ? pgz...
-
Gable Company uses three activity pools. Each pool has a cost driver. Information for Gable Company follows: Activity Pools Total Cost of Pool Machining $ 171,100 Cost Driver Number of machine hours...
-
What is the voltage of a concentration cell of Fe 2+ ions where the concentrations are 0.0025 and 0.750 M ? What is the spontaneous reaction?
-
Calculate the value of the solubility product constant for Cd(OH) 2 from the half-cell potentials.
-
Using the data for the JC Consulting database shown in Figure 2-1, identify the one-to-many relationships as well as the primary key fields and foreign key fields for each of the five tables. TaskID...
-
Outline different applications of digital marketing which can help meet business goals.
-
Give an example of how each of the macro-environment forces may directly drive the content and services provided by a website.
-
What are the market and product positioning opportunities offered by the Internet?
-
The relationship between intermediaries, suppliers and resellers are crucial to every business. Discuss how the Internet potentially changes supply-chain relationships?
-
How should a marketing manager benchmark the online performance of competitors?
-
Refer to the consolidated financial statements of RadioShack Corporation in Appendix B . During 2010, the company had numerous accruals and deferrals. As a new member of RadioShacks accounting staff,...
-
A container holds 2.0 mol of gas. The total average kinetic energy of the gas molecules in the container is equal to the kinetic energy of an 8.0 10-3-kg bullet with a speed of 770 m/s. What is the...
-
A proposed design for a part of a seawall consists of a rectangular solid weighing 3840 lb with dimensions of 8.00 ft by 4.00 ft by 2.00 ft. The 8.00-ft side is to be vertical. Will this object float...
-
A platform is being designed to support some water pollution testing equipment. As shown in Fig. 5.31, its base is 36.00 in wide, 48.00 in long, and 12.00 in high. The entire system weighs 130 lb,...
-
A block of wood with a specific weight of 32 lb/ft 3 is 6 by 6 by 12 in. If it is placed in oil (sg = 0.90) with the 6 by 12-in surface parallel to the surface of the oil, would it be stable?
-
What strategies can be implemented to promote cultural sustainability and preserve endangered languages, traditions, and practices in the face of globalization and rapid social change ?
-
Salter Manufacturing Company produces inventory in a highly automated assembly plant in Fall River, Massachusetts. The automated system is in its first year of operation and management is still...
-
Heart Lake developments sold four lakefront lots for $ 3 0 , 5 0 0 per hectare. If the sizes of the lots in hectares were 3 1 2 , 2 3 7 , 5 5 8 , and 4 4 9 respectively, what was the total sales...

Study smarter with the SolutionInn App


