A mass of 500 lb m of 40 wt% sulfuric acid solution at 140F is diluted with
Question:
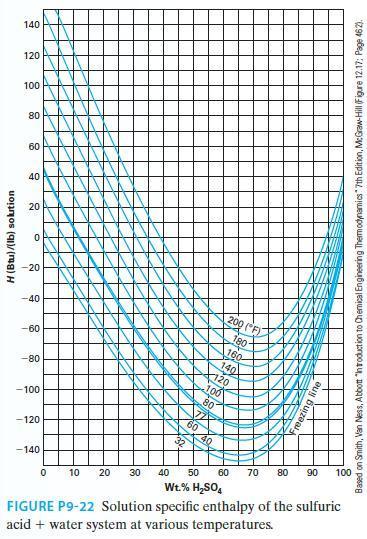
A mass of 500 lbm of 40 wt% sulfuric acid solution at 140°F is diluted with 200 lbm of pure water at 100°F. What is the concentration of the resulting solution? What is the heat effect (liberated or absorbed) of this mixing (report an extensive number) if the mixing is done such that the resulting solution is at 100°F? If the mixing was done adiabatically, what would be the resulting solution temperature? Use Figure P9-22.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





