This sequence of two gas phase reactions can occur in the catalyzed combustion of ammonia: A closed
Question:
This sequence of two gas phase reactions can occur in the catalyzed combustion of ammonia:
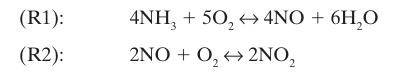
A closed vessel initially contains 5 moles of ammonia and 10 moles of oxygen (Figure 14-2). Assuming these are the only two chemical reactions that occur, write expressions for the mole fractions of each of the species present, in terms of the extents of the two reactions, ₤1 and ₤2
Figure 14‑2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





