We have talked about the diffusivity in liquids and how the Stokes-Einstein formulation works reasonably well. Ionic
Question:
We have talked about the diffusivity in liquids and how the Stokes-Einstein formulation works reasonably well. Ionic liquids are a relatively recent class of liquids useful as replacements for many toxic organic solvents. The diffusivity of gases in these liquids is of interest. We can write a version of Stokes-Einstein using the molar volume of the solute.
\[D\left(\frac{m^{2}}{s}\right) \propto \frac{T}{\mu \bar{V}^{1 / 3}}\]
Where \(T\) is the absolute temperature \((\mathrm{K}), k_{b}\) is Boltzmann's constant, \(\mu\) is the viscosity in \(\mathrm{Pa} \cdot \mathrm{s}\) and \(\bar{V}\) is the molar volume of the gas in \(\mathrm{m}^{3} / \mathrm{kgmol}\). The data below show diffusivities as a function of solvent viscosity for carbon dioxide in a variety of ionic liquids. All data is taken at \(30^{\circ} \mathrm{C}\) where the density of condensed carbon dioxide is \(600 \mathrm{~kg} / \mathrm{m}^{3}\).
a. Plot the data on log-log coordinates and see if the Stokes-Einstein relationship holds.
b. Based on your result from part (a), what is the actual relationship between diffusivity and viscosity for \(\mathrm{CO}_{2}\) in these fluids (i.e., derive a correlation for the diffusivity as a function of viscosity?)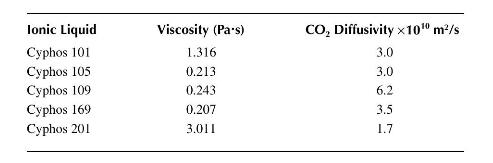
Step by Step Answer:





