The interaction energy between Na + and Cl - ions in the NaCl crystal can be written
Question:
The interaction energy between Na+ and Cl- ions in the NaCl crystal can be written as
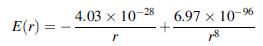
where the energy is given in joules per ion pair and the interionic separation r is in meters. The numerator unit of the first term is J-m and the second term is J-m8. Calculate the binding energy and the equilibrium separation between the Na+ and Cl- ions.
Transcribed Image Text:
E(r) = 4.03 x 10-28 6.97 x 10-9%
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
The interaction energy between Na and Cl ions in the NaCl crystal can be written as Er 403 x 10...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The accompanying graph depicts the interaction energy between two water molecules situated so that their dipole moments are parallel and pointing in the same direction. Sketch an approximate curve...
-
The distance between the K+ and Cl ions in KCl is 2.80 1010 m. Calculate the energy required to separate the two ions to an infinite distance apart, assuming them to be point charges initially at...
-
The distance between the Li+ and Cl ions in LiCl is 0.257 nm. Use this and the molecular mass of LiCl (42.4 g/mol) to compute the density of LiCl.
-
Grimm Company has 2,400,000 shares of common stock outstanding on December 31, 2014. An additional 150,000 shares of common stock were issued on July 1, 2015, and 300,000 more on October 1, 2015. On...
-
Warren Buffett has been a very successful investor. In 2008 Luisa Kroll reported that Buffett topped Forbes Magazine's list of the world's richest people with a fortune estimated to be worth $62...
-
Apples supply chainthe chain of companies from which Apple purchases parts and manufacturing laborincludes over 200 different companies, including some of their own competitors, such as LG and...
-
What is a membrane potential?
-
An iron bar 2.00 cm x 3.00 cm x 10.0 cm at a temperature of 95C is dropped into a barrel of water at 25C. The barrel is large enough so that the water temperature rises negligibly as the bar cools....
-
Light strikes a 6-cm thick Lucite slab of index of refraction 1.56 at an incident angle of 41. The exit point of the light ray is laterally shifted as a result of refraction. Find the distance of...
-
Machinery that cost $192,000 on 1 January 20X1 was sold for $72,000 on 30 June 20X6. It was being depreciated over a 10 year life by the straight line method, assuming its residual value would be...
-
Calculate the total coulombic potential energy of a Na + in a NaCl crystal by considering only up to the fourth nearest neighbors of Na +. The coulombic potential energy for two ions of opposite...
-
Consider the van der Waals bonding in solid argon. The potential energy as a function of interatomic separation can generally be modeled by the Lennard-Jones 612 potential energy curve, that is, E(r)...
-
State in your own words what one means by the statement that y is a function of x.
-
Following are Mill Co.s production costs for October: Direct materials $100,000 Direct labor 90,000 Factory overhead 4,000 What amount of costs should be traced to specific products in the production...
-
When Tyger was 10 years old, his grandparents gave him 5,000 shares of stock in DotCom, Inc. The stock has paid annual dividends of 38,000. This year Tyger's parents have taxable income of $800,000....
-
The inlet contraction and test section of a laboratory wind tunnel are shown. The air speed in the test section is U=50 m/s. A total-head tube pointed upstream indicates that the stagnation pressure...
-
34. SEMO Vases produces the following standard cost to make a clay vase: Direct Materials: 2 pounds @ $4.00 per pound Direct Labor 3 hours @ $9.00 per hour FOH applied at 60% of Direct Labor After...
-
On September 1 of the current year, Scots Company experienced a flood that destroyed the company's entire inventory. Because the company had not completed its month end reporting for August, it must...
-
Let B be an m n matrix of rank n. Show that BTB is positive definite.
-
Sheldon and Leonard had a million-dollar idea. In order to make it happen, they have to do special research first. Only Kripke can help them in this matter. But Kripke is known to be the first-class...
-
We have a 37.0 ( 0.5) wt% HCl solution with a density of 1.18 ( 0.01) g/mL. To deliver 0.050 0 mol of HCl requires 4.18 mL of solution. If the uncertainty that can be tolerated in 0.050 0 mol is 2%,...
-
Compute the molecular mass and its standard uncertainty for NH 3 . What is the percent relative uncertainty in molecular mass?
-
How many significant figures are there in the following numbers? (a) 1.903 0 (b) 0.039 10 (c) 1.40 10 4
-
Question 1 (a) Explain the term "deemed to be included" and its cost implication when used in the Construction Electronic Measurement Standard (CEMS).(6 marks) (b) With the aid of diagrams, identify...
-
Suppose a company has proposed a new 4-year project. The project has an initial outlay of $16,000 and has expected cash flows of $8,000 in year 1, $9,000 in year 2, $12,000 in year 3, and $13,000 in...
-
Mina had 3 times as much money as Nancy at first. After receiving $15 from Nancy, Mina had 5 times as much money as Nancy. (a) (b) How much more money did Mina have than Nancy in the end? Both girls...

Study smarter with the SolutionInn App


