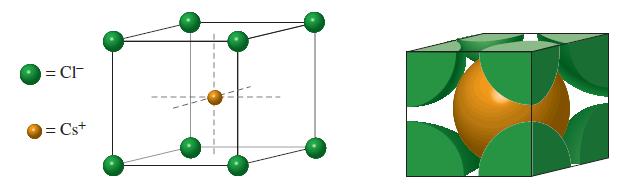
(A) The ionic radius of Cs + is 167 pm. Use Figure 12-49 and information in Examples...
Question:
(A) The ionic radius of Cs+ is 167 pm. Use Figure 12-49 and information in Examples 12-9 and 12-11 to determine the edge length of the unit cell of CsCl.
(B) Use the length of the unit cell of NaCl obtained in Example 12-11, together with the molar mass of NaCl and the Avogadro constant, to estimate the density of NaCl.
Figure 12-49
Examples 12-9
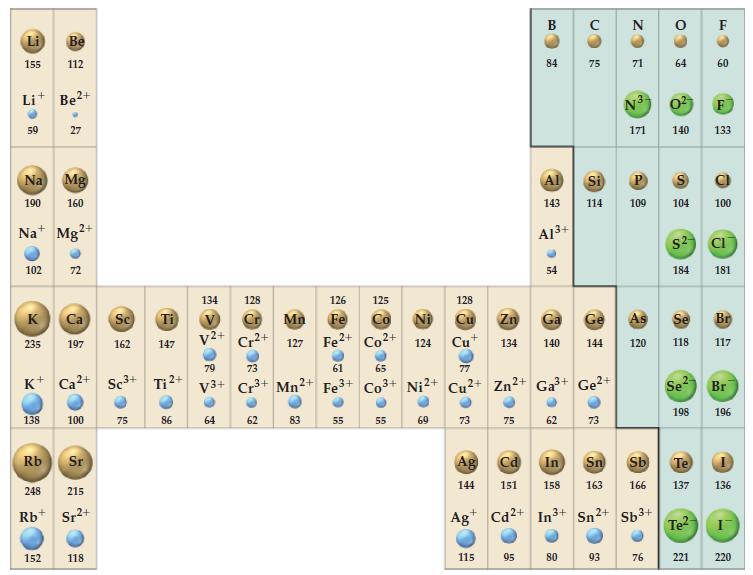
(A) Potassium crystallizes in the bcc structure. What is the length of the unit cell in this structure? Use the metallic radius of potassium given in Figure 9-11.
(B) Aluminum crystallizes in an fcc structure. Given that the atomic radius of Al is 143.1 pm, what is the volume of a unit cell?
Figure 9-11
Examples 12-11
The ionic radii of Na+ and Cl- in NaCl are 99 and 181 pm, respectively. What is the edge length of the unit cell?
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





