Question: An unknown white solid consists of two compounds, each containing a different cation. As suggested in the illustration, the unknown is partially soluble in water.
An unknown white solid consists of two compounds, each containing a different cation. As suggested in the illustration, the unknown is partially soluble in water. The solution is treated with NaOH(aq) and yields a white precipitate. The part of the original solid that is insoluble in water dissolves in HCl(aq) with the evolution of a gas. The resulting solution is then treated with (NH4)2SO4(aq) and yields a white precipitate.
(a) Is it possible that any of the cations Mg2+, Cu2+, Ba2+, Na+, or NH4+ were present in the original unknown? Explain your reasoning.
(b) What compounds could be in the unknown mixture (that is, what anions might be present)?
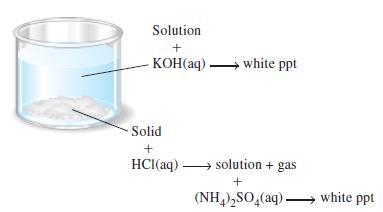
Solution + KOH(aq). Solid + HCl(aq) white ppt solution + gas + (NH4)SO4(aq) white ppt
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
a Yes it is possible that one of the cations Mg2 Cu2 Ba2 Na or NH4 was present in the original unkno... View full answer

Get step-by-step solutions from verified subject matter experts


