By analogy to f H and f G how would you define standard entropy of
Question:
By analogy to ΔfH° and ΔfG° how would you define standard entropy of formation? Which would have the largest standard entropy of formation: CH4(g), CH3CH2OH(l), or CS2(l)? First make a qualitative prediction; then test your prediction with data from Appendix D.
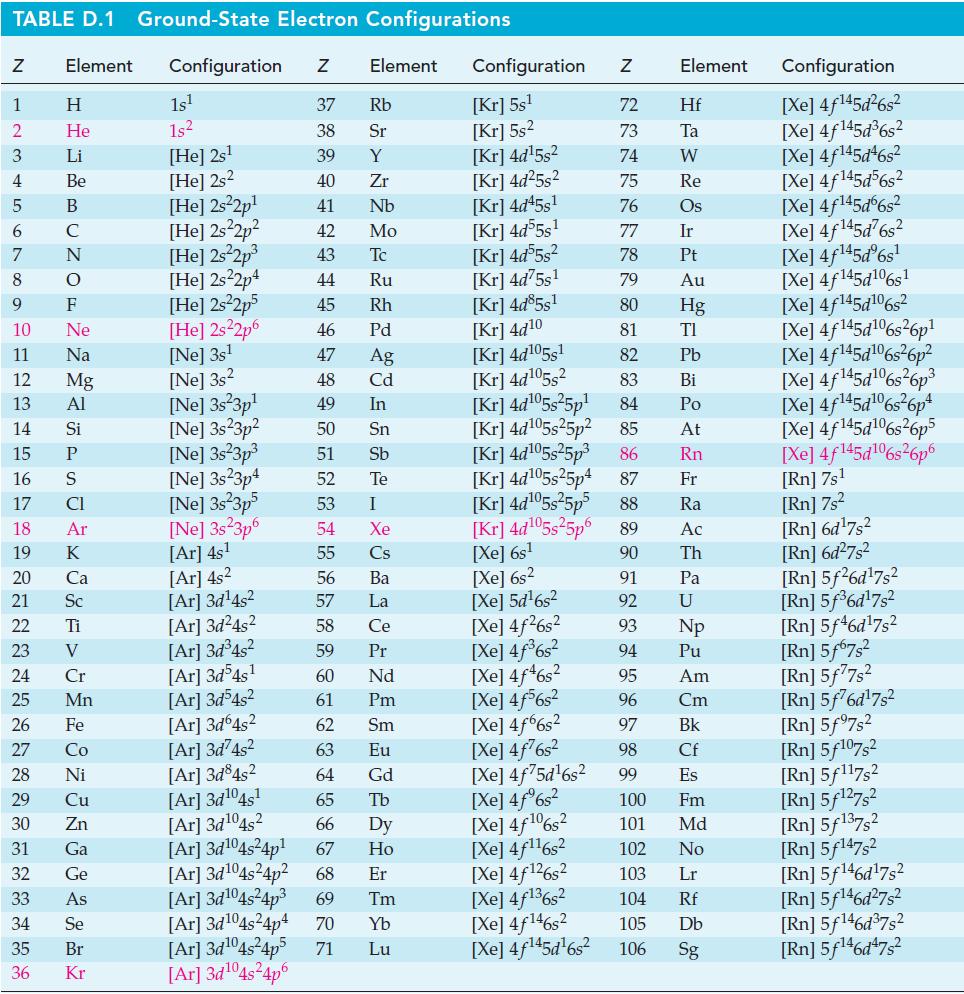
Transcribed Image Text:
TABLE D.1 Ground-State Electron Configurations Element Configuration Z Z 1 2 3 4 5 6 7 8 9 HIGÅ LUZONSUZ SE> 0 ≤ 2 3 2 3 5 3 3 2 2 5 2 He 10 11 12 13 14 15 16 17 18 19 20 21 Sc 22 23 24 25 Mn Mg 26 27 28 Ni 29 30 Zn 31 32 33 34 35 36 Ga Ge 1s¹ 1s² [He] 2s¹ [He] 2s2 [He] 2s²2p¹ [He] 2s²2p² [He] 2s²2p³ [He] 2s22p4 [He] 2s²2p5 [He] 2s²2p6 [Ne] 3s¹ [Ne] 3s2 [Ne] 3s 3p¹ [Ne] 3s23p² [Ne] 3s²3p³ [Ne] 3s23p4 [Ne] 3s²3p5 [Ne] 3s 3p6 [Ar] 4s¹ [Ar] 4s² [Ar]3d¹4s² [Ar] 3d²4s² [Ar] 3d³4s² [Ar] 3d54s¹ [Ar] 3d³4s² [Ar]3d64s² [Ar] 3d²4s² [Ar]3d845² [Ar] 3d¹04s¹ [Ar] 3d¹04s2 [Ar] 3d¹04s²4p¹ Element 37 Rb 38 Sr 39 Y 40 Zr 41 Nb 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 Sb 52 Te 53 54 55 56 57 58 59 60 61 62 63 64 [Ar] 3d¹04s²4p4 [Ar] 3d¹04s²4p5 71 [Ar] 3d¹04s²4p6 I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd 65 Tb 66 67 Dy Ho Er [Ar] 3d¹04s²4p² 68 [Ar] 3d¹04s²4p³ 69 Tm 70 Yb Lu Configuration Z [Kr] 5s¹ [Kr] 5s² [kr] 4d¹5s² [Kr] 4d²5s² [Kr] 4d45s¹ [kr] 4d55s¹ [kr] 4d55s² [Kr] 4d75s¹ [Kr] 4d85s1 [Kr] 4d10 [Kr] 4d105s1 [kr] 4d¹05s² [kr] 4d¹05s²5p¹ [kr] 4d¹05s25p² [Kr] 4d¹05s²5p³ [Kr] 4d¹05s25p4 [Xe] 6s² [Xe] 5d¹6s² [Xe] 4f²6s² [Xe] 4f³6s² [Xe] 4f46s2 [Xe] 4f6s2 [Xe] 4f6s2 [Xe] 4f²6s² [Xe] 4f75d¹6s² [Xe] 4f%s2 [Xe] 4f106s2 [Xe] 4f¹¹6s² NRNKERKR [Xe] 4f126s2 [Xe] 4f136s2 [Xe] 4f146s2 [Xe] 4f¹45d¹6s² 72 Hf 73 Ta W 74 75 Re 76 Os 77 Element 78 79 80 81 82 83 84 85 86 87 Fr [kr] 4d¹05s²5p5 88 Ra [Kr] 4d¹05s²5p6 89 Ac [Xe] 6s¹ 90 Th 91 92 93 94 95 96 97 98 99 Ir Pt Au Hg TI Pb Bi Po At Rn Pa U Np Pu Am Cm Bk Cf Es 100 Fm 101 Md 102 No 103 Lr 104 Rf 105 Db 106 Sg Configuration [Xe] 4f¹45d²6s² [Xe] 4f145d³6s² [Xe] 4f145d46s2 [Xe] 4f145d56s2 [Xe] 4f145d6s2 [Xe] 4f¹45d²6s² [Xe] 4f¹45dº6s¹ [Xe] 4f145d106s1 [Xe] 4f145d106s2 [Xe] 4f145d6s26p* [Xe] 4f145d106s36p? [Xe] 4f145d16s?6p3 [Xe] 4f145d6s®6p* [Xe] 4f145d16s26p5 [Xe] 4f145d106s26p6 [Rn] 7s¹ [Rn] 7s² [Rn] 6d¹7s² [Rn] 6d²7s² [Rn] 5f26d¹7s² [Rn] 5f³6d¹7s² [Rn] 5f46d¹7s2 [Rn] 5f67s² [Rn] 5f77s² [Rn] 5f76d¹7s² [Rn] 5f97s2 [Rn] 5f107,2 [Rn] 5f117s2 [Rn] 5f¹27s² [Rn] 5f137,2 [Rn] 5f147s2 Rn] 5f¹46d¹7s² [Rn] 5f¹46d²7s² [Rn] 5f¹46d³7s2 [Rn] 5f¹46d¹7s²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
How would you define power in your own words? How does power differ from authority? From influence?
-
How would you define leadership? In what are areas of your personal or professional life are you a leader? Which of Jesus leadership traits do you admire most and why? Who do you admire most as a...
-
How would you define uncertain tax position?
-
Find a function (x, y) such that = (y, x).
-
MiniTek manufactures private-label small electronic products, such as alarm clocks, calculators, kitchen timers, stopwatches, and automatic pencil sharpeners. Some of the products are sold as sets,...
-
The Dauten Toy Corporation uses an injection molding machine that was purchased 2 years ago. This machine is being depreciated on a straight-line basis, and it has 6 years of remaining life. Its...
-
In February 2014, defendant Ibrahim M. Shihadeh, d/b/a Creative Designs Kitchen and Baths, agreed to purchase 25% of his anticipated natural gas needs at a fixed price for the 201415 and 201516...
-
John Parsons (123-45-6781) and George Smith (123-45-6782) are 70% and 30% owners, respectively, of Premium, Inc. (11-1111111), a candy company located at 1005 16th Street, Cut and Shoot, TX 77303....
-
Consider two descriptive facts. First, the poorest countries in the world are disproportionately located close to the equator. Second, the civilizations that were richest in 1500 are among the...
-
Use data from Table 13.8 and Figure 13-10 to estimate the temperature at which K = 1.0 x 10 6 for the reaction Table 13.8 Figure 13-10 2 SO2(g) + O2(g) - 2 SO3(g)
-
(A) Estimate the temperature at which K = 5.8 x 10 -2 for the reaction in Example 13-12. Use data from Table 13.8 and Figure 13-10. (B) What is the value of K p for the reaction 2 SO 2 (g) + O 2 (g) ...
-
How did the Affordable Care Act change Medicare Tax withholding percentages?
-
What are the contents of outcome-impact-result statements?
-
Define associations and give examples.
-
How can effectiveness be measured?
-
How is the method of zero-based budgeting carried out?
-
What is the eco-efficiency analysis?
-
According to the wheel of retailing and the retail life cycle, what will happen to factory outlet stores?
-
Suppose that the laptop of Prob. 2.16 is placed in an insulating briefcase with a fully charged battery, but it does not go into sleep mode, and the battery discharges as if the laptop were in use....
-
Supreme Videos, Inc., produces short musical videos for sale to retail outlets. The company?s balance sheet accounts as of January 1, the beginning of its fiscal year, are given on the following...
-
Gold Nest Company of Guandong, China, is a family-owned enterprise that makes birdcages for the South China market. A popular pastime among older Chinese men is to take their pet birds on daily...
-
Almeda Products, Inc., uses a job-order costing system. The companys inventory balances on April 1, the start of its fiscal year, were as follows: During the year, the following transactions were...
-
Requirement 1. Journalize each transaction including explanations. (Record debits first, then credits. Select the explanations on the last line o Apr. 2: Borrowed $49,000 from the bank and signed a...
-
Allen Company sells flags with team logos. Allen Company has fixed costs of $770,000 per year plus variable costs of $11.00 per flag. Each flag sells for $22.00. Read the requirements. Requirement 1....
-
On January 1, 2024, Morey, Incorporated, exchanged $175,225 for 25 percent of Amsterdam Corporation. Morey appropriately applied the equity method to this investment. At January 1, the book values of...

Study smarter with the SolutionInn App


