Cadmium metal is added to 0.350 L of an aqueous solution in which [Cr 3+ ] =
Question:
Cadmium metal is added to 0.350 L of an aqueous solution in which [Cr3+] = 1.00 M. What are the concentrations of the different ionic species at equilibrium? What is the minimum mass of cadmium metal required to establish this equilibrium?
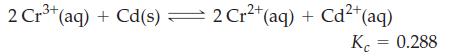
Transcribed Image Text:
2 Cr³+ (aq) + Cd(s) — 2 Cr²+ (aq) + Cd²+ (aq) Kc = 0.288
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The balanced chemical equation for the reaction between cadmium metal and chromiumIII ions is 2Cr3aq ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The energy of the signal g(t) = rect(t)cos(18t) is Select one: O a. None of the answers O b. 1/2 O.1 O d.2
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
An excess of zinc metal is added to 50.0 mL of a 0.100 M AgNO3 solution in a constant-pressure calorimeter like the one pictured in Figure 6.9. As a result of the reaction The temperature rises from...
-
Displacement vector A(vector) points due east and has a magnitude of 2.00 km. Displacement vector B(vector) points due north and has a magnitude of 3.75 km. Displacement vector C(vector) points due...
-
Northwestern Savings and Loan has a current capital structure consisting of $250,000 of 16% (annual interest) debt and 2,000 shares of common stock. The firm pays taxes at the rate of 40%. a. Using...
-
Jeff says to Brenda, I offer to sell you my PC for $900. Brenda replies, If you do not hear otherwise from me by Thursday, I have accepted your offer. Jeff agrees and does not hear from Brenda by...
-
Jerome M. Eisenberg is an antiquities dealer and a self-proclaimed expert in classical antiquities with a doctorate in Roman, Egyptian, and Near Eastern art. Maurice E. Hall Jr. is an art dealer who...
-
Presented below is the ledger for Sparks Co. Instructions (a) Reproduce the journal entries for the transactions that occurred on October 1, 10, and 20, and provide explanations for each. (b)...
-
What are the capabilities of online analytical processing (OLAP)? What is the value of this tool to business? Is OLAP much different from data mining and warehousing? Explain..!
-
One sketch below represents an initial nonequilibrium mixture in the reversible reaction Which of the other three sketches best represents an equilibrium mixture? Explain. 2 NO(g) + Br(g) = 2 NOBr(g)...
-
A mixture of 1.00 mol NaHCO 3 (s) and 1.00 mol Na 2 CO 3 (s) is introduced into a 2.50 L flask in which the partial pressure of CO 2 is 2.10 atm and that of H 2 O(g) is 715 mmHg. When equilibrium is...
-
What are the key differences between T-bills and T-bonds?
-
Identify a number of changes in consumer behavior expected to impact upon the digital healthcare industry AND critically assess their potential impact - on industry and on society.
-
At present, 100,000 tourists visit a city per year, spending an average of $500 per visit. The government is considering a subsidy removing the toll on a highway entering the city for visitors (not...
-
You are delinquent on one of your credit cards. You have agreed with the bank to make payments of $90 per month starting the end of this month. The interest rate on the balance is 1% per month. If...
-
List and explain the criteria that should be used when investing an organization's cash in the short term.
-
Evluate f 10x(x + 3) dx
-
PB, Inc. manufactures and sells a popular line of fat-free cookies under the name of Aunt May. The process PB uses to manufacture the cookies is labor-intensive; it relies heavily on direct labor....
-
Explain five different cases of income exempt from tax with clear examples.
-
Prepare an income statement through gross profit for Norris Company using the variance data in Practice Exercises 23-1A, 23-2A, 23-3A, and 23-4A. Assume Norris sold 500 units at $105 per unit.
-
Prepare an income statement through gross profit for McLean Company using the variance data in Practice Exercises 23-1B, 23-2B, 23-3B, and 23-4B. Assume McLean sold 2,500 units at $214 per unit.
-
The following are inputs and outputs to the cooking process of a restaurant: Percent of meals prepared on time Number of unexpected cook absences Number of times ingredients are missing Number of...
-
3. The carrying amount of property owned by X Ltd at the end of the year amounted to $216,000. On this date the property was revalued to fair value of $190,000. The balance on the revaluation reserve...
-
Mustique Health Inc is considering an investment of $1,550,000 in a new diagnostic equipment to detect lung health. The equipment is expected to have a salvage value equal to 8% of the original...
-
This is my current code. I need help on why the math is not working properly. The output is completely off for some reason. Please explain as to what I can do better with my code as well. #include...

Study smarter with the SolutionInn App


