One sketch below represents an initial nonequilibrium mixture in the reversible reaction Which of the other three
Question:
One sketch below represents an initial nonequilibrium mixture in the reversible reaction
![]()
Which of the other three sketches best represents an equilibrium mixture? Explain.
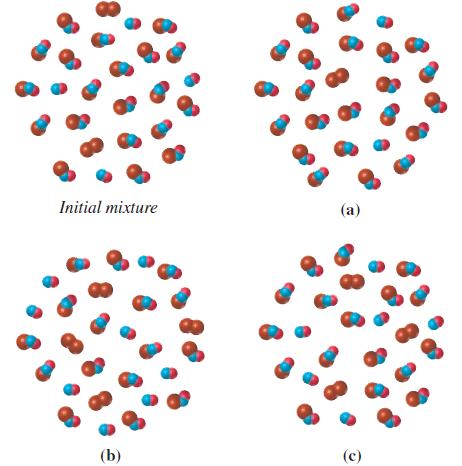
Transcribed Image Text:
2 NO(g) + Br₂(g) = 2 NOBr(g) Kc = 3.0
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Have regulatory changes occurring since 2000 had a significant positive or negative impact on the manner in which board of directors operate? Explain
-
One sketch below represents an initial nonequilibrium mixture in the reversible reaction Which of the other three sketches best represents an equilibrium mixture? Explain. Kc = 4.0 (8) (8) + (8)
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Assume your company shows the market values of equity and debt at the level of $175373 and $224626, respectively. The rate of return on assets is 33 percent and its volatility is 45 percent. The...
-
Max Small has outstanding school loans that require a monthly payment of $1,000. He needs to purchase a new car for work and estimates that this will add $350 per month to his existing monthly...
-
Barnes accepted Clarks offer to sell to him a portion of Clarks coin collection. Clark forgot that his prized $20 gold piece at the time of the offer and acceptance was included in the portion which...
-
Fantastic Sams and Defendants PSTEVO, LLC and Jeremy Baker entered into a franchise agreement pursuant to which Fantastic Sams granted PSTEVO a franchise to operate a Fantastic Sams Salon. According...
-
On the basis of your answers to Problems 25-1 and 25-2, if Hastings were to acquire Vandell, what would be the range of possible prices that it could bid for each share of Vandell common stock?...
-
Write a three page paper on Compare and contrast Online Analytic Processing (OLAP) and Online Transaction Processing (OLTP); also discuss Codds rules for TP databases and OLAP databases. Cite any...
-
Lead metal is added to 0.100 M Cr 3+ (aq). What are [Pb 2+ ], [Cr 2+ ], and [Cr 3+ ] when equilibrium is established in the reaction? 3+ Pb(s) + 2 Cr+ (aq) = Pb+ (aq) + 2 Cr+ (aq) K 3.2 x 10-10 =
-
Cadmium metal is added to 0.350 L of an aqueous solution in which [Cr 3+ ] = 1.00 M. What are the concentrations of the different ionic species at equilibrium? What is the minimum mass of cadmium...
-
Loratadine, marketed as an antiallergy medication under the name Claritin, has four rings, eight double bonds, and the formula C 22 H ? C1N 2 O 2 . How many types of hydrogen does loratadine have?...
-
Flywheels are large, massive wheels used to store energy. They can be spun up slowly, then the wheel's energy can be released quickly to accomplish a task that demands high power. An industrial...
-
A stockroom worker pushes a box with mass 10.6 kg on a horizontal surface with a constant speed of 3.30 m/s . The coefficients of kinetic and static friction between the box and the surface are 0.310...
-
You plan to deposit $5,607 into a savings account paying 6% interest compounded quarterly. How much will be in your account - assuming no withdrawals-after three years?
-
Could you please explain internal electric field formation at Ohmic contact for example for n - type semiconductor, to which direction electric field points?
-
Determine the air temperature in Celsius if the speed of sound (in air) is 388.38m/s Recall T [C] +273 = T [K]
-
Pilgrim Corporation makes a range of products. The company's predetermined overhead rate is $23 per direct labor-hour, which was calculated using the following budgeted data: Variable manufacturing...
-
Is the modified 5-question approach to ethical decision making superior to the modified moral standards or modified Past in approach?
-
McLean Company produced 2,500 units of product that required two standard hours per unit. The standard fixed overhead cost per unit is $1.30 per hour at 4,600 hours, which is 100% of normal capacity....
-
Norris Company produced 500 units that require six standard pounds per unit at $1.25 standard price per pound. The company actually used 2,900 pounds in production. Journalize the entry to record the...
-
McLean Company produced 2,500 units that require three standard gallons per unit at $18.50 standard price per gallon. The company actually used 8,000 gallons in production. Journalize the entry to...
-
Write a Python program requesting a name and three numbers from the user. The program will need to calculate the following: the sum of the three numbers the result of multiplying the three numbers...
-
The Finance Department at Salt Lake City International Airport (SLC) is forecasting next year's budget for parking lot snow plowing expenses. They would like to compare the total number of seats...
-
choose a website that is visit frequently by college students.Determine one item from the list below that this website does welland one item that islacking.Detail each and discuss your point of view....

Study smarter with the SolutionInn App


