Equations (1) and (2) can be combined to yield the equation for the formation of CH 4
Question:
Equations (1) and (2) can be combined to yield the equation for the formation of CH4(g) from its elements.
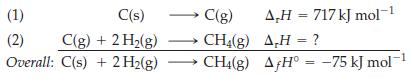
Use the preceding data and a bond energy of 436 kJ mol-1 for H2 to estimate the C—H bond energy. Compare your result with the value listed in Table 10.3.
Table 10.3
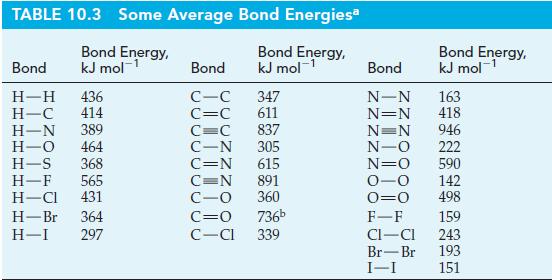
Transcribed Image Text:
C(s) C(g) (1) (2) C(g) + 2 H₂(g) →→→→ CH4(g) Overall: C(s) + 2 H2(g) → CH4(g) 1 A,H = 717 kJ mol-¹ AH = ? AƒH° = -75 kJ mol-¹
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
AH 717 KJmol Cs C g Cg 2H28 CH4g AH7...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A pipe is to be carried around the right- angled corner of two intersecting corridors. Suppose that the widths of the two intersecting corridors are 5 feet and 8 feet ( see Figure 6- 26). Your...
-
In Exercises 2930, use nine pixels in a 3 3 grid and the color levels shown. Write a 3 3 matrix that represents a digital photograph of the letter T in dark gray on a light gray background. White...
-
The standard enthalpy of formation of H 2 O 2 (g) is -136 kJ mol -1 . Use this value, with other appropriate data from the text, to estimate the oxygen-to-oxygen single-bond energy. Compare your...
-
Graph the solution sets in Problems 316. x 100
-
Second Chance Welding rebuilds spot welders for manufacturers. The following budgeted cost data for 2014 is available for Second Chance. The company desires a $30 profit margin per hour of labor and...
-
Determine the height h of block B so that the rod is in neutral equilibrium. The springs are unstretched when the rod is in the vertical position. The block has a weightW. B.
-
On December 14, 2011, appellant Aaron Olson contracted to receive telephone service from respondent CenturyLink and also applied for reduced-rate service that CenturyLink provides through Minnesotas...
-
In analyzing legal expense for the Boastman Bottle Company, Mary Little, CPA, observes that the company has paid legal fees to three different law firms during the current year. In accordance with...
-
2. A muon is created in our upper atmosphere and travels toward the ground at 0.998 c. If it's lifetime is 2.2 x 10-6 seconds. What is the lifetime of the muon as observed by the earth's frame of...
-
A reaction involved in the sequence of reactions leading to the destruction of ozone is Estimate the oxygen-oxygen bond energy in ozone by using the oxygenoxygen bond energy in dioxygen from Table...
-
Use bond energies from Table 10.3 to estimate r H for the following reaction. Table 10.3 CH2(g) + H2(g) CH4(g) AH = ?
-
Referring to Table 12-6, what is the 90% confidence interval for the expected change in average heating costs as a result of a 1 degree Fahrenheit change in the daily minimum outside temperature...
-
AdamCo agrees to sell the latest version of its Go! video game to Cutter Game stores. AdamCo delivers an outdated version of Go! (nonconforming goods). Cutters possible remedies may include a....
-
Alto Corporation agrees to buy ten saxophones from Musical Equipment Warehouse (MEW). When MEW fails to deliver, Alto is forced to cover. Alto sues MEW. Alto can recover from MEW a. the cover price...
-
To disclaim the implied warranty of merchantability, a merchant must mention merchantability. (True/False)
-
Tyler Desk Corporation writes in its contracts, in large red letters, There are no warranties that extend beyond the description on the face hereof. The disclaimer negates a. the implied warranty of...
-
Noels Ski Shop sells a pair of skis to Verlyn. When he first uses the skis, they snap in two. The cause is something that Noel did not know about and could not have discovered. If Verlyn sues Noel,...
-
The Morning Brew Coffee Shop sells Regular, Cappuccino, and Vienna blends of coffee. The shop's current daily labor cost is $320, the equipment cost is $125, and the overhead cost is $225. Daily...
-
A spacecraft has left the earth and is moving toward Mars. An observer on the earth finds that, relative to measurements made when the spacecraft was at rest, its a. length is shorter b. KE is less...
-
Theory of constraints, throughput contribution, quality. Refer to the information in Exercise 19-25 in answering the following requirements. There is no connection between the requirements. 1....
-
Quality improvement, relevant costs, and relevant revenues. The Thomas Corporation sells 300,000 V262 valves to the automobile and truck industry. Thomas has a capacity of 110,000 machine-hours and...
-
Quality improvement relevant costs, and relevant revenues. The Tan Corporation uses multicolor molding to make plastic lamps. The molding operation has a capacity of 200,000 units per year. The...
-
Culver Hardware Limited reported the following amounts for its cost of goods sold and inventory: 2024 2023 Cost of goods sold $167,000 $155,400 Ending inventory 37,600 30,000 Culver made two errors...
-
The books of Waterway Corporation carried the following account balances as of December 31, 2025. Cash $190,000 Preferred Stock (6% cumulative, nonparticipating, $50 par) 316,000 Common Stock (no-par...
-
A taxpayer has $50,000 in machinery to depreciate, $10,000 of which is qualified reuse and recycling property, placed into service in TY2023. $10,000 of the machinery is used; the rest is new. How...

Study smarter with the SolutionInn App


