Show that for nonstandard conditions the temperature variation of a cell potential is where E(T 1 )
Question:
Show that for nonstandard conditions the temperature variation of a cell potential is
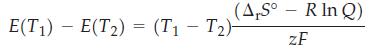
where E(T1) and E(T2) are the cell potentials at T1 and T2 respectively. We have assumed that the value of Q is maintained at a constant value. For the nonstandard cell below, the potential drops from 0.394 V at 50.0 °C to 0.370 V at 25.0 °C Calculate Q, ΔrH°, and ΔrS° for the reaction, and calculate K for the two temperatures.
![]() Choose concentrations of the species involved in the cell reaction that give the value of Q that you have calculated, and then determine the equilibrium concentrations of the species at 50.0 °C.
Choose concentrations of the species involved in the cell reaction that give the value of Q that you have calculated, and then determine the equilibrium concentrations of the species at 50.0 °C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





