Show that for a combination of half-cell reactions that produce a standard reduction potential for a half-cell
Question:
Show that for a combination of half-cell reactions that produce a standard reduction potential for a half-cell that is not directly observable, the standard reduction potential is
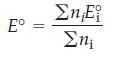
where is the number of electrons in each half-cell reaction of potential Ei°. Use the following half-reactions:
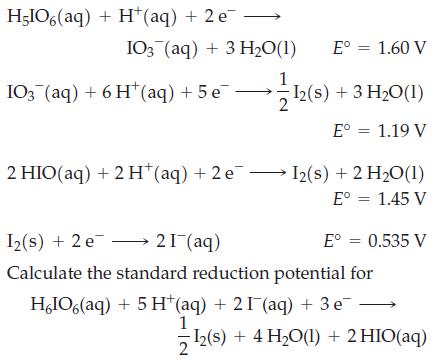
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





