The following data are from a laboratory experiment that examines the relationship between solubility and thermodynamics. In
Question:
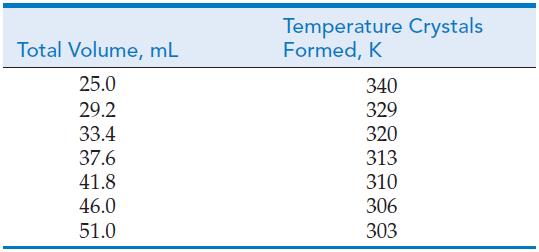
The following data are from a laboratory experiment that examines the relationship between solubility and thermodynamics. In this experiment KNO3(s) is placed in a test tube containing some water. The solution is heated until all the KNO3(s) is dissolved and then allowed to cool. The temperature at which crystals appear is then measured. From this experiment we can determine the equilibrium constant, Gibbs energy, enthalpy, and entropy for the reaction. Use the following data to calculate ΔrG, ΔrH, and ΔrS for the dissolution of KNO3(s). (Assume the initial mass of KNO3(s) was 20.2 g.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





