Thermite mixtures are used for certain types of welding, and the thermite reaction is highly exothermic. 1.00
Question:
Thermite mixtures are used for certain types of welding, and the thermite reaction is highly exothermic.
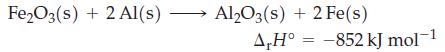
1.00 mol of granular Fe2O3 and 2.00 mol of granular Al are mixed at room temperature (25 °C), and a reaction is initiated. The liberated heat is retained within the products, whose combined specific heat capacity over a broad temperature range is about 0.8 J g-1 °C-1. (The melting point of iron is 1530 °C.) Show that the quantity of heat liberated is more than sufficient to raise the temperature of the products to the melting point of iron.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





