Thymol blue in its acid range is not a suitable indicator for the titration of HCl by
Question:
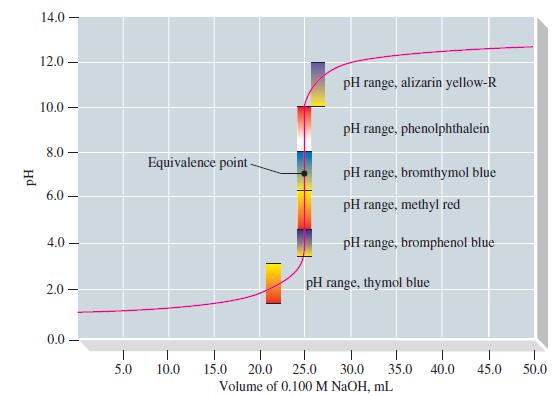
Thymol blue in its acid range is not a suitable indicator for the titration of HCl by NaOH. Suppose that a student uses thymol blue by mistake in the titration of Figure 17-8 and that the indicator end point is taken to be pH = 2.0.
(a) Would there be a sharp color change, produced by the addition of a single drop of NaOH(aq)?
(b) Approximately what percent of the HCl remains unneutralized at pH = 2.0?
Figure 17-8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





