Question: Use data from Appendix D to determine values at 298 K of and K for the following reactions. (The equations are not balanced.) (a) HCl(g)
Use data from Appendix D to determine values at 298 K of and K for the following reactions. (The equations are not balanced.)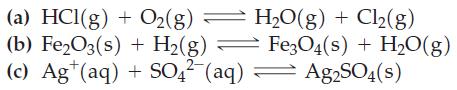
(a) HCl(g) + O(g) (b) FeO3(s) + H(g) (c) Ag(aq) + SO4 (aq) HO(g) + Cl(g) Fe3O4(s) + HO(g) Ag2SO4(s)
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
To determine the values of G and K for the given reactions at 298 K we can use the following equatio... View full answer

Get step-by-step solutions from verified subject matter experts


