Question: Use data from Table 7.2 and r H for the following reaction to determine the standard enthalpy of formation of hexane, C 6 H
Use data from Table 7.2 and ΔrH° for the following reaction to determine the standard enthalpy of formation of hexane, C6H14(l), at 25 °C and 1 bar.
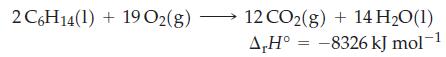
Table 7.2
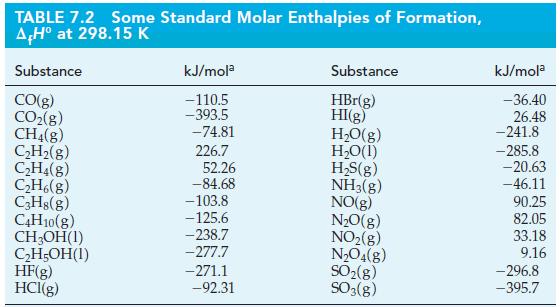
2 C6H14(1) + 19 O2(g) 12 CO(g) + 14 HO(1) A,H -8326 kJ mol-1
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
To determine the standard enthalpy of formation of hexane C6H14l at 25 C and 1 bar using data fr... View full answer

Get step-by-step solutions from verified subject matter experts


