Use standard enthalpies of formation from Tables 7.2 and 7.3 and equation (7.22) to determine the standard
Question:
Use standard enthalpies of formation from Tables 7.2 and 7.3 and equation (7.22) to determine the standard enthalpy of reaction in the following reaction.
![]()
Tables 7.2
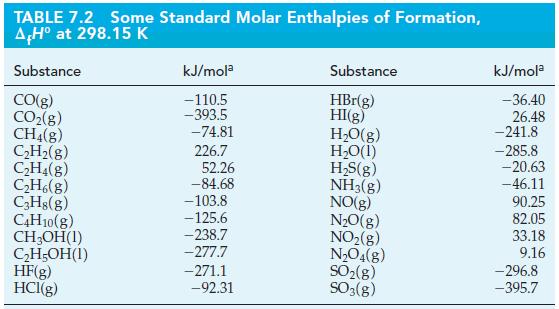
Tables 7.3
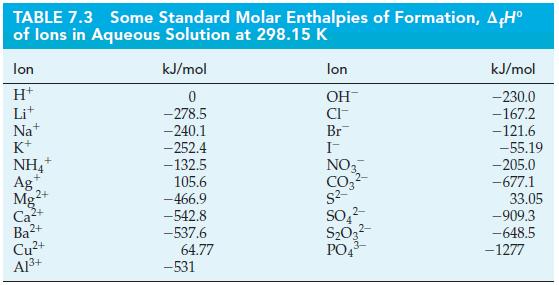
Eq. 7.22![A,H [cx AHc + dx AHD + = weighted sum of A H values for the products .] [ax AHA + bx AfH B + weighted sum of](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/0/4/555654de84b5bbd41699604550819.jpg)
Transcribed Image Text:
NH4+ (aq) + OH(aq) H₂O(1) + NH3(g).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of reaction in the following reactions. Table 7.2 Eq. 7.22 (a) C3H8(g) + H(g) CH6(g) +...
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
(a) Why are tables of standard enthalpies of formation so useful? (b) What is the value of the standard enthalpy of formation of an element in its most stable form? (c) Write the chemical equation...
-
Tom Jones, the mechanic at Golden Muffler Shop, is able to install new mufflers at an average rate of 4 per hour (or about 1 every 15 minute), according to a negative exponential distribution....
-
Laredo Boot Company makes specialty boots for the rodeo circuit. On December 31, 2008, the company had (a) 300 pairs of boots in finished goods inventory and (b) 1,400 heels at a cost of $16 each in...
-
Based on Exhibit 1, the PVDBO is closest to: A . $3,585 million. B . $3,633 million. C . $3,681 million.
-
In the past five years, there have been significant innovations in technology such as smartphones and tablets. Technology companies rely on intellectual property (IP) rights, such as patents,...
-
Myers Company provides you with the following condensed balance sheet information. For each transaction below, indicate the dollar impact (if any) on the following five items: (1) total assets, (2)...
-
About a year and a half ago, your parents purchased some appliances at a major retailer, taking advantage of a "no payments and no interest offer. Under the agreement, for 18 months no interest would...
-
Putt Corporation acquired 70 percent of Slice Companys voting common stock on January 1, 20X3, for $158,900. Slice reported common stock outstanding of $100,000 and retained earnings of $85,000. The...
-
The standard enthalpy of fermentation of glucose to ethanol is Use the standard enthalpy of combustion for glucose to calculate the enthalpy of combustion for ethanol. C6H12O6(s)- 2 CH3CHOH(1) + 2...
-
One glucose molecule, C 6 H 12 O 6 (s), is converted to two lactic acid molecules, CH 3 CH(OH)COOH(s) during glycolysis. Given the combustion reactions of glucose and lactic acid, determine the...
-
Assume that Temp Force is a constant growth company whose last dividend (D0, which was paid yesterday) was $2.00 and whose dividend is expected to grow indefinitely at a 6%rate. What is the firms...
-
Sardar, an agent in FIA, has recently seized illegal weapons valued at USD 10 million. As a reward for his services, PM Imran Khan has offered him a sizeable bonus. Sardar is planning to invest his...
-
A store is considering carrying a new product which will require an upfront purchase of $125,000 for inventory. Free cash flows expected as a result of this project are shown below. At the end of the...
-
5. Firm ABC is expecting a significant growth in revenue in the next few years. Before conducting a financial forecast, Firm ABC would like to analyze its internal growth rate and external funding...
-
A 15-year bond has a coupon rate of 4.25% and a yield to maturity of 3.2%. The bond pays coupons semi-annually. What is the fair price of the bond?
-
Question 1 3 pts In the video "Professionalism in Writing," I tell a story from the Olympics. What was the basic story? What is its significance in terms of how we define professionalism?
-
Warmers Dress Shop had net retail sales of $250,000 during the current year. The following additional information was obtained from the companys accounting records: 1. Using the retail method,...
-
Catalytic hydrogenation of naphthalene over PdC results in rapid addition of 2 moles of H 2 . Propose a structure for this product.
-
Indicate how the following items are recorded in the accounting records in the current year of Coronet Co. (a) Impairment of goodwill. (b) A change in depreciating plant assets from accelerated to...
-
Whittier Construction Co. had followed the practice of expensing all materials assigned to a construction job without recognizing any salvage inventory. On December 31, 2010, it was determined that...
-
Parsons Inc. wishes to change from the completed-contract to the percentage-of-completion method for financial reporting purposes. The auditor indicates that a change would be permitted only if it is...
-
Given two vectors A = 3.80 +7.20) and B-5.00 -2.20j, find the scalar product of the two vectors A and B. 20 ? A. B= Submit Request Answer 4 Part B Find the angle between these two vectors. Express...
-
Vector A is in the direction 39.0 clockwise from the -y-axis. The x-component of A is A = -18.0 m. Part A What is the y-component of A? Express your answer with the appropriate units. u A 0 ? Ay = -...
-
Air conditioning for a college dormitory will cost $2.1 million to install and $170,000 per year to operate. The system should last 19 years. The real cost of capital is 9%, and the college pays no...

Study smarter with the SolutionInn App


