Write cell reactions for the electrochemical cells diagrammed here, and use data from Table 19.1 to calculate
Question:
Write cell reactions for the electrochemical cells diagrammed here, and use data from Table 19.1 to calculate E°cell for each reaction.
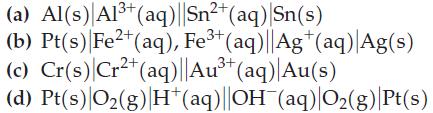
Table 19.1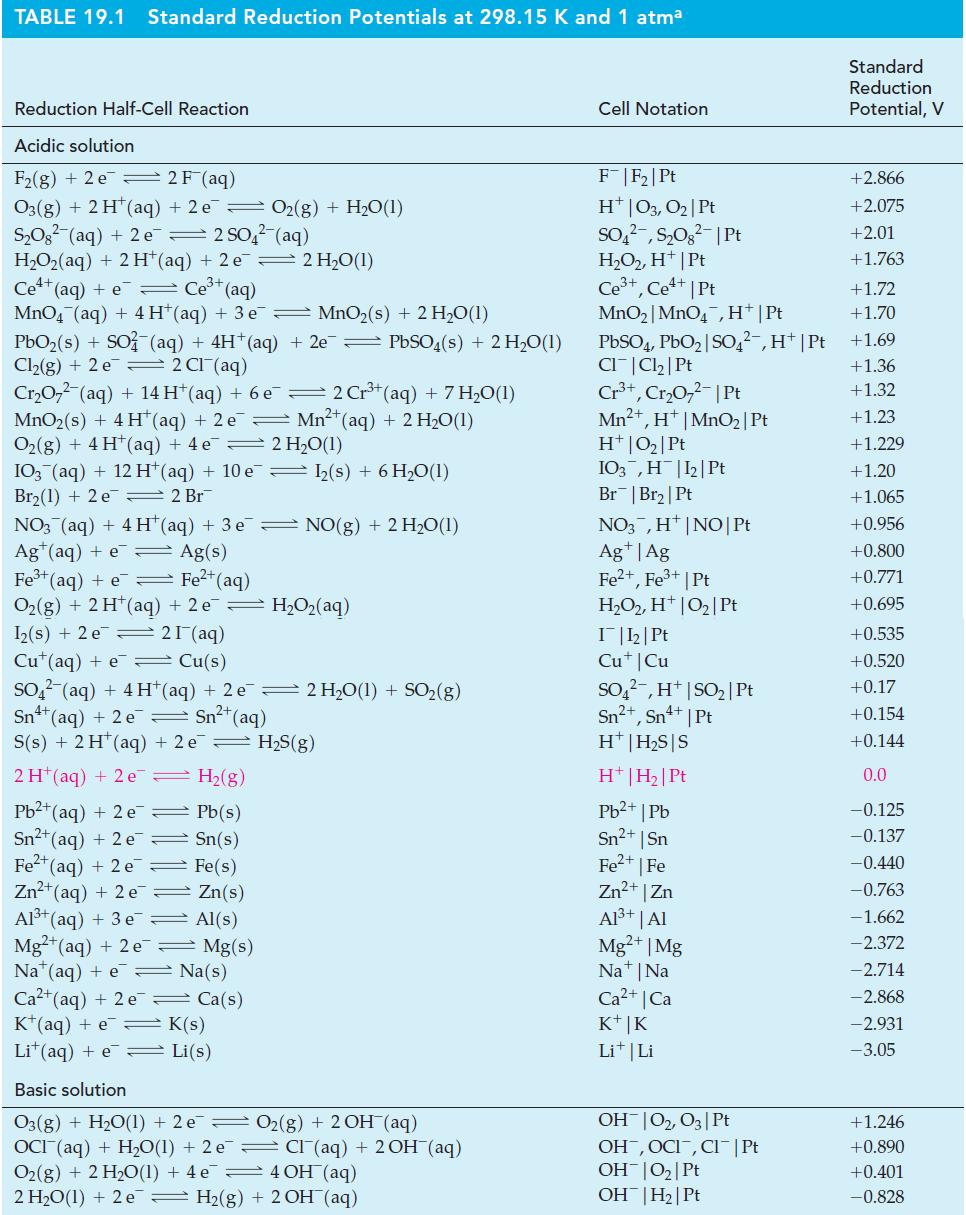
Transcribed Image Text:
2+ (a) Al(s) Al³+ (aq)||Sn²+ (aq)|Sn(s) (b) Pt(s) Fe2+ (aq), Fe³+ (aq)||Ag+ (aq) Ag(s) (c) Cr(s) Cr²+ (aq)||Au³+ (aq)| Au(s) 2+ 3+ (d) Pt(s) O₂(g) H*(aq)||OH¯(aq)|O₂(g) |Pt(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Write the half-cell reactions and the balanced chemical equation for the electrochemical cells diagrammed here. Use data from Table 19.1 and Appendix D to calculate E cell for each reaction. Table...
-
(A) Use data from Table 19.1 to predict the probable products when Pt electrodes are used in the electrolysis of KI(aq). Table 19.1 (B) In the electrolysis of AgNO 3 (aq), what are the expected...
-
Use the table below to find: (fog)(-10) = (fof) (11) = X -10 f(x) -1 g(x) 4 -1 -7 -4 -7 11 (gof)(-4)= (gog)(7) = 4 7 15 4 7 -4 11 -4 15 7 -1 15 -10 -7 -10 11
-
93) Clay of the Land is a manufacturer of glazed clay pots. Currently, it produces 300 clay pots per month which it sells through nurseries at a constant price of $5 per pot. Current demand for clay...
-
Discuss the various methods of overcoming blocked channels.
-
Briefly define what an ethical dilemma is and describe the steps to consider when evaluating ethical dilemmas.
-
Faculty Classroom Hours The dean of a university estimates that the mean number of classroom hours per week for full-time faculty is 11.0. As a member of the student council, you want to test this...
-
An engineering student bought a car at a local used car lot. Including tax and insurance, the total price was $3000. He is to pay for the car in 12 equal monthly payments, beginning with the first...
-
Tyo and U 1 F Costs Under Perpetual Inventory System The following units of an item were available for sale during the year: \ table [ [ Beginning inventory, 2 1 , 6 0 0 units at $ 2 0 . 0 0
-
Use the data in Appendix D to calculate the standard cell potential for each of the following reactions. Which reactions will occur spontaneously? (a) H(g) + F2 (g) (b) Cu(s) + Ba+ (aq) 2 H(aq) + 2 F...
-
Consider the reaction Co(s) + Ni 2+ (aq) Co 2+ (aq) + Ni(s), with E cell = 0.02 V. If Co(s) is added to a solution with [Ni 2+ ] = 1 M, should the reaction go to completion? Explain.
-
The (X, Y) coordinates (in feet) for a closed-polygon traverse ABCDEFA follow A (1000.00, 1000.00), B (1661.73, 1002.89), C (1798.56, 1603.51), D (1289.82, 1623.69), E (1221.89, 1304.24) and F...
-
As a project manager it is important to utilize the right tool at the right time. When it comes to managing quality on projects, this is no exception. Identify three 'Total Quality Tools' that you...
-
Describe 2 change models that you could use to create change in an organization. Choose 1 of the models that you think would be most successful in an organization, and analyze reasons why you chose...
-
During the current year, Rothchild, Inc., purchased two assets that are described as follows. Heavy Equipment Purchase price, $375,000. Expected to be used for 10 years, with a residual value at the...
-
Regarding the Mozilla case, assume that Communities of Practice start to arise spontaneously around topics that are related to the visualizations in the Portal at Mozilla. What do you think is the...
-
Regarding Issues That Affect Recruitment, how would you proceed as the assistant superintendent for human resources in a school district that is experiencing a shortage of qualified applicants for...
-
The following is a portion of the Statement of Shareholders' Equity from Cisco Systems' January 25, 2014, quarterly report. Remember that comparative purposes, three years are reported in these...
-
Clark, PA, has been engaged to perform the audit of Kent Ltd.s financial statements for the current year. Clark is about to commence auditing Kents employee pension expense. Her preliminary enquiries...
-
Columbia Sportswear Company had accounts receivable of $206,024,000 at the beginning of a recent year, and $267,653,000 at year-end. Sales revenues were $1,095,307,000 for the year. What is the...
-
Young Corporation reported income taxes of $340,000,000 on its 2010 income statement and income taxes payable of $277,000,000 at December 31, 2009, and $522,000,000 at December 31, 2010. What amount...
-
Flynn Corporation reports operating expenses of $80,000 excluding depreciation expense of $15,000 for 2010. During the year prepaid expenses decreased $6,600 and accrued expenses payable increased...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


