Use the method of Exercise 75 to determine the volume of 0.1000 M KMnO 4 required to
Question:
Use the method of Exercise 75 to determine the volume of 0.1000 M KMnO4 required to titrate 5.00 mL samples of H2O2(aq) for each of the entries in Table 20.1. Plot these volumes of KMnO4(aq) as a function of time, and show that from this graph you can get the same rate of reaction at 1400 s as that obtained in Figure 20-2.
Exercise 75
Exactly 300 s after decomposition of H2O2(aq) begins (reaction 20.3), a 5.00 mL sample is removed and immediately titrated with 37.1 mL of 0.1000 M KMnO4. What is at this 300 s point in the reaction?
Reaction (20.3)
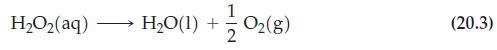
Table 20.1
![TABLE 20.1 Decomposition of HO2 Time, s 0 200 400 600 1200 1800 3000 [HO2], M 2.32 2.01 1.72 1.49 0.98 0.62](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/3/2/12965622a210c72e1700932127357.jpg)
Figure 20-2
![[H2O2], mol 2.50 - 2.00 - 1.50 - 1.00- 0.50- I 400 800 1200 1600 Time, s 2000 I 2400 2800](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/3/2/15565622a3b2c1011700932153434.jpg)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





