The initial rate of reaction (20.3) is found to be 1.7 x 10 -3 M s -1
Question:
The initial rate of reaction (20.3) is found to be 1.7 x 10-3 M s-1. Assume that this rate holds for 2 minutes. Start with 175 mL of 1.55 M H2O2(aq) at t = 0. How many milliliters of O2(g), measured at 24°C and 757 mmHg, are released from solution in the first minute of the reaction?
Reaction (20.3)
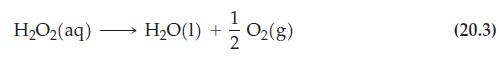
Transcribed Image Text:
H,Oz(aq) H₂O(1) + 1/02 (8) (20.3)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
ANSWER To determine the amount of O2 released in the first minute of the reaction we need to use the ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Googles ease of use and superior search results have propelled the search engine to its num- ber one status, ousting the early dominance of competitors such as WebCrawler and Infos- eek. Even later...
-
For the reaction I (aq) + OCl (aq) OI (aq) + Cl (aq) occurring in aqueous solution, the following mechanism has been proposed: a. Derive the rate law expression for this reaction based on this...
-
Eva pays $3,000 per month to rent her house. She has a garage but is considering turning the space into a hair styling studio. She expects to earn $3,000 a month from this new business. Instead, she...
-
Predict your results. Write "yes" or "no," depending on whether you think the plate will show growth. Give the reason (s) for your predictions. Observe the colonies through the petri plate lids. Do...
-
Describe the component approach to computing depreciation.
-
List three reasons for creating a logical data flow diagram.
-
Sometimes people who act inappropriately are just trying to save their jobs and the company. How do you feel about those types of persons?
-
The comparative balance sheet of Rowe Products Inc. for December 31, 2013 and 2012, is as follows: The income statement for the year ended December 31, 2012, is as follows: The following additional...
-
Write a C program that asks the user to enter the sum and difference of 2 integer numbers. The program will find the 2 numbers and display them as shown below. Input validation: sum plus difference...
-
We have seen that the units of k depends on the overall order of a reaction. Derive a general expression for the units of k for a reaction of any overall order, based on the order of the reaction (o)...
-
Use the method of Exercise 75 to determine the volume of 0.1000 M KMnO 4 required to titrate 5.00 mL samples of H 2 O 2 (aq) for each of the entries in Table 20.1. Plot these volumes of KMnO 4 (aq)...
-
Using data from Exercise 43, determine the molarity of O 2 in an aqueous solution at equilibrium with air at normal atmospheric pressure. The volume percent of O 2 in air is 20.95%. Exercise 43 Under...
-
Cramer Corp. issued $20,000,000 of 5-year, 9% bonds at a market (effective) interest rate of 10%, receiving cash of $19,227.757. Interest on the bonds is payable semiannually. Journalize the entry to...
-
A 4.5-kg, three legged stool supports a 65-kg person. If each leg of the stool has a cross-sectional diameter of 1.8 cm and the weight of the person is evenly distributed, determine the pressure...
-
Cavy Company estimates that total factory overhead costs will be $777,924 for the year. Direct labor hours are estimated to be 102,900. a. Compute the predetermined factory overhead rate. Round your...
-
Find the present value of the following cash flow: receive $7 every year for 28years with the first payment being 9 years from now. Assume an effective annual rate of 5.1%
-
Briefly describe LABU. Include the history of the company, its products and its customers. Explain why you chose this particular company and your projection for its future growth. Include applicable...
-
Johnson Company uses the allowance method to account for uncollectible accounts receivable. Bad debt expense is established as a percentage of credit sales. For 2011, net credit sales totaled...
-
Below is a sample of the data in the file NFLAttendance which contains the 32 teams in the National Football League, their conference affiliation, their division, and their average home attendance....
-
What is the normal balance for each of the following accounts? (a) Accounts Receivable. (b) Cash. (c) Owners Drawing. (d) Accounts Payable. (e) Service Revenue. (f) Salaries Expense. (g) Owners...
-
Indicate whether each of the following accounts is an asset, a liability, or an owners equity account and whether it has a normal debit or credit balance: (a) Accounts Receivable, (b) Accounts...
-
For the following transactions, indicate the account debited and the account credited. (a) Supplies are purchased on account. (b) Cash is received on signing a note payable. (c) Employees are paid...
-
Certified Internal Auditor (CIA) - Certifying body is the Institute of Internal Auditors https://na.theiia.org/certification/CIA-Certification/Pages/CIA-Certification.aspx CIA Certification...
-
Company A has the following data: Prime costs are 180% of direct materials costs. DM costs for year 20xx are $ 100,000. The company had estimated the overhead costs to be $400,000 and the estimated...
-
Using the AICPA Code of Professional Conduct Links to an external site. describe how this was either applied, misapplied, or completely ignored. Using the COSO Internal ControlIntegrated Framework...

Study smarter with the SolutionInn App


