Question: Write K sp expressions for the following equilibria. For example, for the reaction AgCl(s) Ag + (aq) + Cl - (aq), K sp =
Write Ksp expressions for the following equilibria. For example, for the reaction AgCl(s) ⇌ Ag+(aq) + Cl-(aq), Ksp = [Ag+][Cl-].
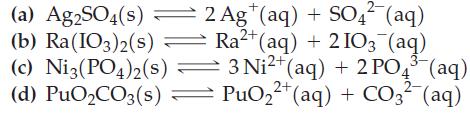
(a) Ag2SO4(s) (b) Ra(103)2(s) 2 Ag+ (aq) + SO4 (aq) Ra+ (aq) + 2103 (aq) (c) Ni3(PO4)2(s) 3 Ni+ (aq) + 2 PO4(aq) 2+ 2- (d) PuOCO3(s) = PuO+ (aq) + CO3- (aq)
Step by Step Solution
★★★★★
3.51 Rating (164 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The solubility product constant Ks... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


