Shown below is a diagram depicting the enthalpy change of a chemical reaction run at constant pressure.
Question:
Shown below is a diagram depicting the enthalpy change of a chemical reaction run at constant pressure.
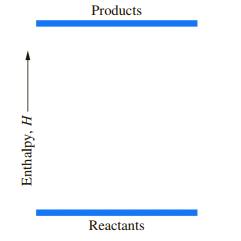
a. Is the reaction exothermic or endothermic?
b. What is the sign of ∆H?
c. What is the sign of q?
d. If the reaction does no work, what is the sign of ∆E for this process?
Transcribed Image Text:
Products Reactants Enthalpy, H-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
a Because the enthalpy increases when going from rea...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Thermodynamics and Thermochemistry 105 [Useful information : 1 J= 1kg m's-2, 1 Pa = 1 kg m s; 1 bar 10 Pa] Given that AS (A +C) = 50 eu AS C + D) = 30 eu AS (D - B) = -20 euwhere, eu is entropy unit...
-
14 Thermodynamics and Thermochemistry . The reaction, MgO(s) + C(s) Mg(s) + CO(g ) 18 The entropy change associated with the conversion of 1 kg of ice at 273 K to water vapours at 383 K is for which...
-
1. Which has maximum internal energy at 298 K? a) helium gas c) ozone gas d) equal b) oxygen gas For a gas having molar mass M, specific heat at constant pressure can be given as: YR a) M(Y-1) YRM a...
-
In Exercises find the derivative of the function by the limit process. f(x) = x - 4x + 5
-
The file Energy contains the per capita energy consumption, in kilowatt-hours, for each of the 50 states and the District of Columbia during a recent year. a. Compute the mean, variance, and standard...
-
Maaz has a bank account with less than $400 in it. His bank pays no interest on that account and charges $2 per month for each month the account is below $500. How much will be in Maazs account if he...
-
We see that 75 of the 264 people in the study allowed the pressure to reach its maximum level of \(300 \mathrm{mmHg}\), without ever saying that the pain was too much (MaxPressure=yes). Use this...
-
Minelli Enterprises uses large amounts of copper in the manufacture of ceiling fans. The firm has been very concerned about the detrimental impact of rising copper prices on its earnings and has...
-
What are the key components of a knowledge management framework designed to facilitate tacit knowledge transfer within a complex organizational ecosystem ?
-
Cooperative San Jos of southern Sonora State in Mexico makes a unique syrup using cane sugar and local herbs. The syrup is sold in small bottles and is prized as a flavoring for drinks and for use in...
-
Hydrogen sulfide, H 2 S, is produced during decomposition of organic matter. When 0.5000 mol H 2 S burns to produce SO 2 (g) and H 2 O(l), 281.0 kJ of heat is released. What is this heat in...
-
Consider the following specific heats of metals. Metal .......................Specific Heat copper...................... 0.385 J/(gC) magnesium ...............1.02 J/(gC) mercury...
-
What are the reasons/motivations behind the exponential growth of social media analytics?
-
Common stock value-Variable growth Personal Finance Problem Home Place Hotels, Inc., is entering into a 3-year remodeling and expansion project. The construction will have a limiting effect on...
-
How do recombinant DNA techniques contribute to the production of biopharmaceuticals, vaccines, and industrial enzymes through microbial fermentation, mammalian cell culture, and transgenic organism...
-
what ways does the interdisciplinary nature of recombinant DNA technology foster collaborations between molecular biologists, biochemists, engineers, and clinicians to address pressing societal...
-
How do advancements in synthetic biology and genome editing technologies, such as CRISPR-Cas systems and base editing tools, expand the capabilities of recombinant DNA technology to engineer complex...
-
Explain why non-integrated sales and marketing information systems lead to enterprise-wide inefficiencies, higher costs, lost profits, and customer dissatisfaction?
-
Between early 2008 and the beginning of 2009, a gradual stock-market downturn and plummeting home prices generated a substantial reduction in U.S. household wealth that induced most U.S. residents to...
-
Gopher, Inc. developing its upcoming budgeted Costs of Quality (COQ) with the following information: Expense Item Budget Raw Materials Inspection $ 15,000 EPA Fine 200,000 Design Engineering 15,000...
-
A vessel containing 39.5 cm3 of helium gas at 25oC and 106 kPa was inverted and placed in cold ethanol. As the gas contracted, ethanol was forced into the vessel to maintain the same pressure of...
-
The volume occupied by a gas depends linearly on degrees Celsius at constant pressure, but it is not directly proportional to degrees Celsius. However, it is directly proportional to kelvins. What is...
-
A sample of 62.3 cm3 of argon gas at 18oC was contained at a pressure of 155 kPa in a J shaped tube with mercury. Later the temperature changed. When the mercury level was adjusted to give the same...
-
Write the feature of Super's Career Development Assessment and Counseling (C-DAC) system?
-
Describe one type of role-play technique you could use in a specific group setting. Explain who would benefit from this technique and why. Toseland, R. W., & Rivas, R. F. (2017). An introduction to...
-
Suppose that a manager is following a base stock policy where the optimal inventory position is 10. Assume the component lead time is 2 days. At the end of day 1, there is no ordered units yet to be...

Study smarter with the SolutionInn App


