A plant manufacturing dry ice will burn coke in air to produce a flue gas which, when
Question:
A plant manufacturing dry ice will burn coke in air to produce a flue gas which, when cleaned and cooled, will contain \(15 \% \mathrm{CO}_{2}, 6 \% \mathrm{O}_{2}\), and \(79 \% \mathrm{~N}_{2}\). The gas will be blown into a bubble-cap tower scrubber at \(1.2 \mathrm{~atm}\) and \(298 \mathrm{~K}\), to be scrubbed countercurrently with a \(30 \mathrm{wt} \%\) monoethanolamine \(\left(\mathrm{C}_{2} \mathrm{H}_{7} \mathrm{ON}\right)\) aqueous solution entering at \(298 \mathrm{~K}\). The scrubbing liquid, which is recycled from a stripper, will contain \(0.058 \mathrm{~mol} \mathrm{CO}_{2} / \mathrm{mol}\) solution. The gas leaving the scrubber is to contain \(2 \% \mathrm{CO}_{2}\). A liquid-to-gas ratio of 1.2 times the minimum is specified. Assume isothermal operation. At \(298 \mathrm{~K}\) and \(1.2 \mathrm{~atm}\), the equilibrium mol fraction of carbon dioxide over aqueous solutions of monoethanolamine ( \(30 \mathrm{wt} \%\) ) is given by
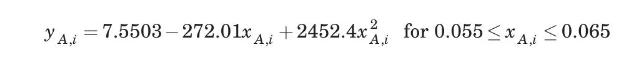
where \(x_{A, i}\) is the mol fraction of \(\mathrm{CO}_{2}\) in the liquid solution.
(a) Calculate the kilograms of solution entering the tower per cubic meter of entering gas.
(b) Determine the number of theoretical trays required for part (a).
(c) The monoethanolamine solution has a viscosity of \(6.0 \mathrm{cP}\) and a density of \(1012 \mathrm{~kg} / \mathrm{m}^{3}\). Estimate the overall tray efficiency for the absorber, and the number of real trays required. Seader and Henley (1998) proposed the following empirical correlation to estimate the overall efficiency of absorbers and strippers using bubble-cap trays (it has also been used to obtain rough estimates for sieve-tray towers):
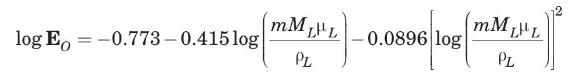
where \(\quad \mathbf{E}_{O}=\) overall fractional efficiency \(m=\) slope of equilibrium curve \(\mu_{L}=\) liquid viscosity, in \(\mathrm{cP}\)
\(ho_{L}=\) liquid density, in \(\mathrm{kg} / \mathrm{m}^{3}\)
Step by Step Answer:






