(a) In aqueous solution at pH 0, Mn 3+ disproportionates to MnO 2 and Mn 2+ ....
Question:
(a) In aqueous solution at pH 0, Mn3+ disproportionates to MnO2 and Mn2+. Write equations for the two half-reactions involved in this process.
(b) Use Fig. 8.2 to obtain values of Eº for the half-equations in part (a).
(c) Determine Eºcell and a value of ΔGº(298 K) for the disproportionation of Mn3+ (aq) at pH 0. Write an equation to which this value of ΔGº(298 K) refers.
Figure 8.2.
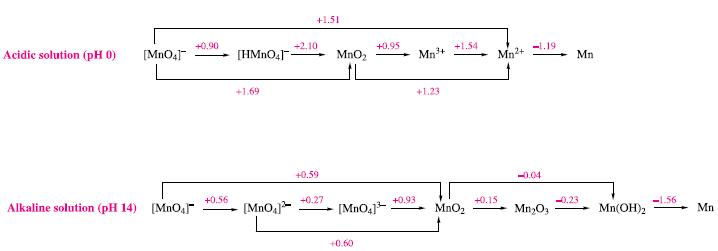
Transcribed Image Text:
Acidic solution (pH 0) [MnO4] Alkaline solution (pH 14) [MnO4] +0.90 +0.56 [HMnO4] +1.69 [MnO4] +2.10 +1.51 +0.59 +0.27 MnO₂ +0.95 [MnO4]³ +0.60 +0.93 Mn³+ +1.23 +1.54 MnO₂ Mn²+ +0.15 -1.19- -0.04 Mn₂O3 Mn -0.23 Mn(OH)2 -1.56 Mn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a The disproportionation of Mn3 in aqueous solution at pH 0 can be represented by the following half...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Use appropriate data from Appendix 11 to determine the ratio of the overall stability constants of the complexes [Fe(phen) 3 ] 2+ and [Fe(phen) 3 ] 3+ at 298 K. (b) Use the data in Fig. 8.2 to...
-
A chemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. The drug molecules bind the protein in a...
-
27. A company has 2 machines that produce widgets. An older machine produces 23% defective widgets, while the new machine produces only 8% defective widgets. In addition, the new machine produces 3...
-
In Problem perform the indicated operations and reduce answers to lowest terms. 2 4 ? 16 x? + 4x
-
California Housewares Merced Division is a small manufacturer of wooden household items. Al Rivkin, divisional controller, plans to implement a standard-costing system. Rivkin has collected...
-
Write a networking objective.
-
Stone Brewing Co. is a San Diego brewer that has sold its beers for over two decades. Stone has maintained its trademark and brand from the beginning, registering the STONE mark in 1998. Stone has...
-
Copa Corporations accountant left for vacation before completing the monthly cost variance report. The corporations president has asked you to complete the report. The following data are available...
-
To determine the kinetics (rates) of ozone depletion reactions, chemists perform controlled laboratory studies. In this simulated lab, we will interpret data obtained from such laboratory experiments...
-
(a) With the aid of a phase diagram, explain what is meant by a supercritical fluid. Give examples of commercial processes that involve the use of supercritical fluids. (b) Even though CO 2 is...
-
Suggest likely products for the following reactions (which are balanced on the left-hand sides) in liquid NH 3 . How does reaction (d) differ from the behaviour of MeCO 2 H in aqueous solution? (a)...
-
A group of residents were asked about their support for a homeless shelter being opened in their neighborhood. They were also asked about how long they had lived in the neighborhood, with short-term...
-
Lou owns two hundred acres next to Brooks lumber mill. Lou sells to Brook the privilege of removing timber from his land to cut into lumber. The privilege of removing the timber is a. an easement. b....
-
Kaiden sells his house and yard to Jill. When Jill arrives to take possession, she learns that Kaiden has removed the kitchen cabinets from the house and the plastic lawn furniture from the yard....
-
Sloan operated ChoCo, a gourmet chocolate factory. When the business doubled and then tripled in size, Sloan wanted to expand ChoCos facilities. To accomplish the expansion, Sloan needed to buy fifty...
-
James Heal owned a vehicle salvage yard in Homestead, Iowa. Brian Anderson contracted with Heal to run the business. Anderson cleaned up the property, removed trash, installed heat and fixed the...
-
Tony and Julie Odigie took out an adjustable-rate mortgage from Nationstar Mortgage, LLC, to purchase their new home. They were late with the first payment and delinquent several times a year for...
-
On August 1, Year 1, Zip Ltd. purchased some merchandise from a foreign company for DM450,000. The liability was not due until March 1, Year 2. Zip was quite confident that the exchange rate...
-
A simple random sample of 220 university students were asked what pasta they usually order and with which sauce. The preferences of these respondents are summarised below: Sauce Bolognese Pasta...
-
Which member of the following pairs is the stronger acid? Give reasons for your choice. (a) [Fe(OH 2 ) 6 ] 3+ or [Fe(OH 2 ) 6 ] 2+ , (b) [Al(OH 2 ) 6 ] 3+ or [Ga(OH 2 ) 6 ] 3+ , (c) Si(OH) 4 or...
-
Use Paulings rules to place the following acids in order of increasing acid strength: HNO 2 , H 2 SO 4 , HBrO 3 , and HClO 4 in a nonlevelling solvent.
-
Arrange the following ions in order of increasing acidity in aqueous solution: Fe 3+ , Na + , Mn 2+ , Ca 2+ , Al 3+ , Sr 2+ .
-
Kane Biotech was preparing the annual financial statements and, as part of the year-end procedures, assessed the assets and prepared the following alphabetized schedule based on adjusted values at...
-
Can you elucidate the interplay between genetic imprinting, genomic imprinting disorders, and cloning, highlighting the challenges associated with preserving parent-specific epigenetic marks and...
-
Workplace bullying is a phenomenon that has been receiving increasing attention in recent years. Workplace bullying can not only affect the immediate victim but also have ripple effects within the...

Study smarter with the SolutionInn App


