Question: Using data from Table 8.1, write down the spontaneous cell process, and calculate E o cell and G o for the following combinations of half-cells:
Using data from Table 8.1, write down the spontaneous cell process, and calculate Eocell and ΔGo for the following combinations of half-cells:
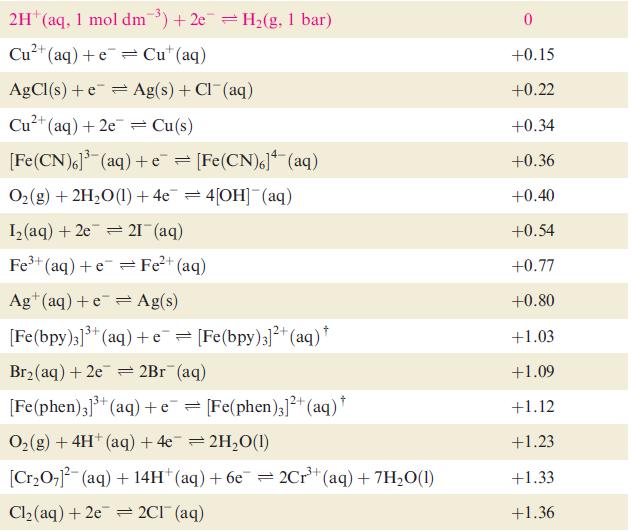
Data from Table 8.1
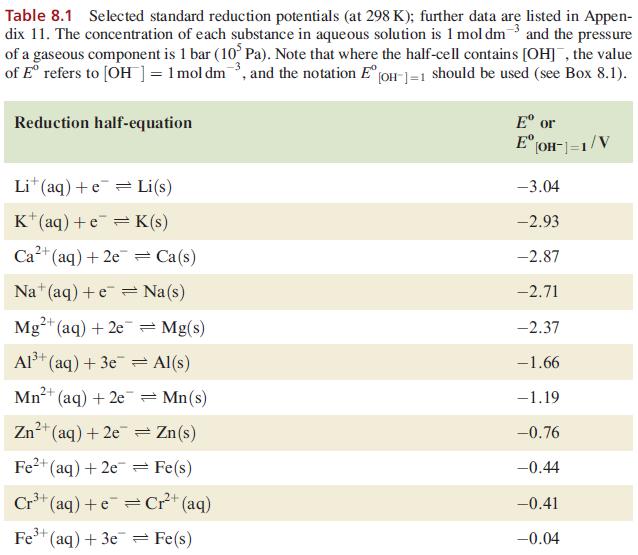
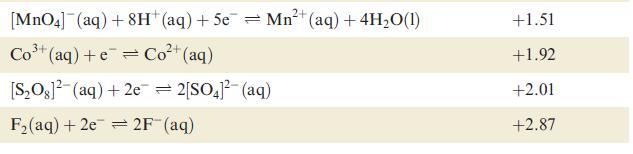
(a) Ag+ (aq) +e=Ag(s) (b) Br (aq) + 2e = 2Br (aq) 2+ with Zn+ (aq) + 2e = Zn(s) with Cl(aq) + 2e = 2Cl(aq) (c) [CrO (aq) + 14H+ (aq) + 6e = 2 Cr+ (aq) +7HO(1) with Fe+ (aq) + e = Fe+( + (aq) 3+
Step by Step Solution
3.34 Rating (160 Votes )
There are 3 Steps involved in it
To solve this problem we need to identify the halfcell reactions and use the given standard reduction potentials E to write down the spontaneous cell ... View full answer

Get step-by-step solutions from verified subject matter experts


