Ethanol(1) + benzene(2) form azeotropic mixtures. (a) From the limited data below at 45 C, it is
Question:
Ethanol(1) + benzene(2) form azeotropic mixtures.
(a) From the limited data below at 45 °C, it is desired to estimate the constant A for the one-term Margules equation, GE/RT = Ax1x2. Use all of the experimental data to give your best estimate.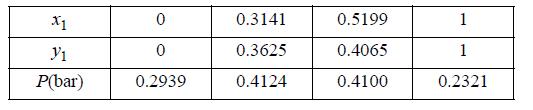
(b) From your value, what are the bubble pressure and vapor compositions for a mixture with x1 = 0.8?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





