Robert Boyle (1627-1691) established the law that (pressure (times) volume) (=) constant for a gas at a
Question:
Robert Boyle (1627-1691) established the law that (pressure \(\times\) volume) \(=\) constant for a gas at a constant temperature. By pouring mercury into the open top of the long side of a \(\mathrm{J}\)-shaped tube, he increased the pressure on the air trapped in the short leg. The volume of trapped air \(=h \times\) cross section, where \(h\) is the height of the air in the short leg. If \(y=\) height of mercury, adjusted for the pressure of the atmosphere on the open end, then \(y\) and \(x=1 / h\) should obey a straight-line relationship.
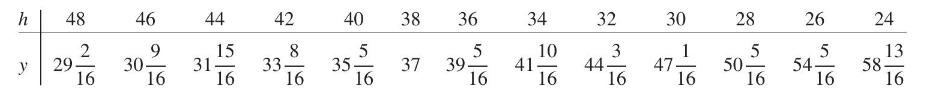
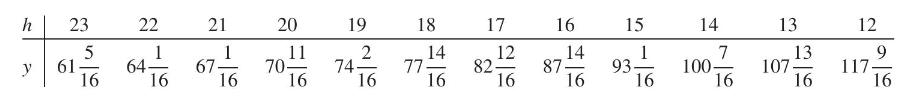
(a) Fit a straight line by least squares to Boyle's data.
(b) Check the residuals for a possible violation of the assumptions.
Step by Step Answer:

Probability And Statistics For Engineers
ISBN: 9780134435688
9th Global Edition
Authors: Richard Johnson, Irwin Miller, John Freund





