Question: In Problem 8.8 we found that the trial wave function with shielding (Equation 8.28), which worked well for helium, is inadequate to confirm the existence
In Problem 8.8 we found that the trial wave function with shielding (Equation 8.28), which worked well for helium, is inadequate to confirm the existence of a bound state for the negative hydrogen ion. Chandrasekhar used a trial wave function of the form
![]()
where
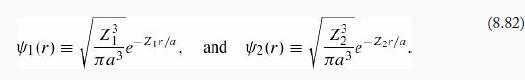
In effect, he allowed two different shielding factors, suggesting that one electron is relatively close to the nucleus, and the other is farther out. (Because electrons are identical particles, the spatial wave function must be symmetrized with respect to interchange. The spin state—which is irrelevant to the calculation—is evidently antisymmetric.) Show that by astute choice of the adjustable parameters Z1 and Z2 you can get (H) less than -13.6 eV.
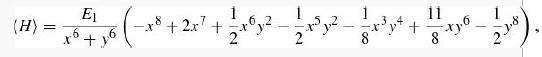
where x Ξ Z1 + Z2 and y Ξ 2√Z1Z2. Chandrasekhar used Z1 = 1.039 (since this is larger than 1, the motivating interpretation as an effective nuclear charge cannot be sustained, but never mind—it’s still an acceptable trial wave function) and Z2 = 0.283.
(r, r2) = A[V1 (1)2(12) + 2(1)(2)], (8.81)
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
where The integral is the same as in Eq 821 only with a 4 Z 1 Z 2 a C... View full answer

Get step-by-step solutions from verified subject matter experts


