In Chapter 12, you learned that when metal ions dissolve in water they become solvated (hydrated), meaning
Question:
In Chapter 12, you learned that when metal ions dissolve in water they become solvated (hydrated), meaning they form ion–dipole attractions to many water molecules (often six). For example, Na+(aq) really looks like this in water:
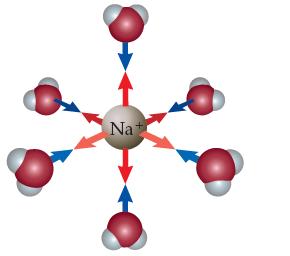
Fe3+ (aq) ions also do this, but unlike Na+(aq) ions, they also make the solution acidic. The reason is that one of the bound water molecules can donate a proton to a free water molecule in the solvent. Draw a picture like the one above for Fe3+ (aq), and show how Fe3+ (aq) makes water acidic.
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:




