Sodium bicarbonate has the formula NaHCO 3 , and in solution, it gives aqueous Na+ ions and
Question:
Sodium bicarbonate has the formula NaHCO3, and in solution, it gives aqueous Na+ ions and aqueous HCO3– (bicarbonate) ions.
(a) Is the HCO3– ion the conjugate base of carbonic acid (H2CO3)? (H2CO3 is a weak acid.) Explain.
(b) Is the HCO3– ion the conjugate acid of carbonic ion (CO32–)? [CO32– is a weak base.] Explain.
(c) If you dissolve NaHCO3 in water, will the resulting solution be acidic or basic? [The following information should help you decide.]
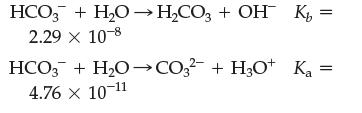
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





