Question: Use the periodic table to predict an ionic charge for each of the following metal ions. (a) Li ion (b) Mg ion (c) Al ion
Use the periodic table to predict an ionic charge for each of the following metal ions.
(a) Li ion
(b) Mg ion
(c) Al ion
(d) Sn ion.
Periodic Table
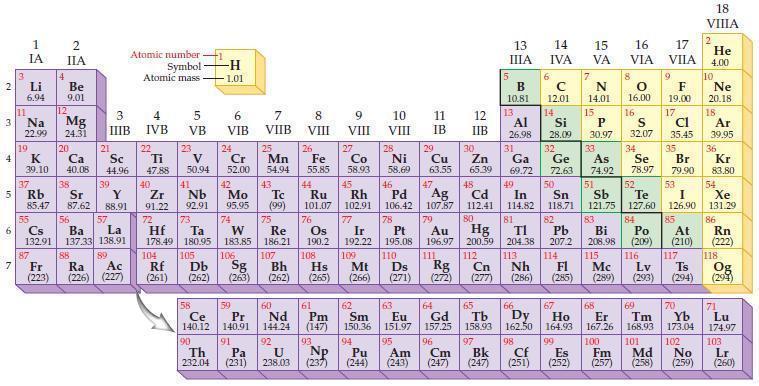
2 3 4 5 6 7 3 11 Li 6.94 1 IA Na 22.99 19 37 al 55 4 87 2 IIA Fr (223) Be 9.01 12 K Ca Sc 39.10 40.08 44.96 Mg 24.31 38 Rb Sr Y 85.47 87.62 88.91 56 57 Cs Ba La 132.91 137.33 138.91 20 21 88 3 IIIB 39 89 Atomic number Symbol - Ac Ra (226) (227) Atomic mass 4 IVB 22 Ti 47.88 40 5 VB 23 V 50.94 41 Zr Nb 91.22 92.91 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 104 105 Rf Db (261) (262) (263) Sg 106 59 Pr 140.91 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 Fe Co 55.85 58.93 Os 190.2 44 45 Ru Rh 101.07 102.91 76 108 9 VIII 61 Pm (147) 27 Bh Hs Mt (262) (265) (266) 93 U NP 238.03 (237) 77 Ir 192.22 109 10 VIII 62 Sm 150.36 94 28 Ni 58.69 46 Pd 106.42 78 11 IB Pt 195.08 110 29 Cu 63.55 13 IIIA 12 IIB 5 B 10.81 13 17 16 VA VIA VIIA 8 Al 26.98 6 C 12.01 14 Si 28.09 32 30 31 33 As Se Br Zn Ga Ge 65.39 69.72 72.63 74.92 78.97 79.90 14 15 IVA 7 N 14.01 15 P 30.97 16.00 83 Bi 208.98 115 16 S 32.07 34 84 47 48 49 50 51 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 79 Au Hg 196.97 200.59 111 112 Rg Cn (271) (272) (277) (286) (285) (289) (293) (294) 81 82 TI Pb 204.38 207.2 113 114 Nh Fl Ds Mc Lv Ts Po (209) 9 116 F 19.00 Md (258) 17 Cl 35.45 35 53 I 126.90 85 At (210) 66 67 69 70 63 64 65 68 Eu Gd Tb Dy Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 95 101 102 Pu Am (244) (243) Cm (247) 97 Bk (247) 98 99 100 Cf Es Fm (251) (252) (257) 117 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
In general the ionic charge of a metal ion can be predicted by looking at the metals group number ve... View full answer

Get step-by-step solutions from verified subject matter experts


