Question: Predict the ionic charge for a chlorine ion, Cl ?- , based on the position of the element in the periodic table. Periodic Table: 2
Predict the ionic charge for a chlorine ion, Cl?-, based on the position of the element in the periodic table.
Periodic Table:
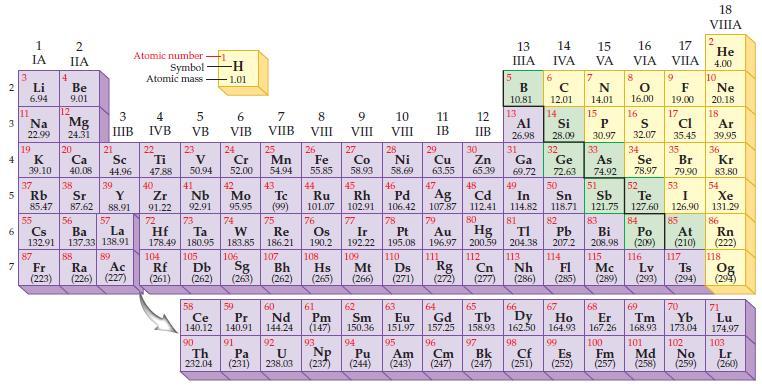
2 3 4 5 6 7 Li 6.94 11 1 IA Na 22.99 19 37 55 4 2 IIA 87 Be 9.01 12 K Ca 39.10 40.08 Mg 24.31 20 38 21 Rb Sr Y 85.47 87.62 88.91 88 Sc 44.96 3 4 IIIB IVB 39 56 Cs La Ba 132.91 137.33 138.91 57 89 Atomic number - Symbol Atomic mass Fr Ra Ac (223) (226) (227) 22 Ti 47.88 40 Zr 91.22 72 104 5 VB Rf (261) 23 V 50.94 41 Nb 92.91 Hf Ta 178.49 180.95 73 105 58 Ce 140.12 90 -H 1.01 Th 232.04 6 VIB 24 Cr 52.00 Db Sg (262) (263) 106 42 Tc Mo 95.95 (99) 74 59 Pr 140.91 7 VIIB 75 Re 183.85 186.21 91 25 Pa (231) Mn 54.94 43 107 Bh (262) 60 Nd 144.24 92 8 VIII 26 Fe 55.85 7 8 O 16.00 16 13 N 14.01 15 Al Si P S Cl 26.98 28.09 30.97 32.07 35.45 17 32 35 33 34 Ge As Se Br 72.63 74.92 78.97 79.90 50 Ag 82 51 52 53 Sn Sb Te I 118.71 121.75 127.60 126,90 83 84 Pb Bi Po 207.2 208.98 (209) 85 107.87 79 Pt Au 195.08 196.97 110 111 Ds 108 204.38 113 Nh 115 116 Rg Cn 114 FI Mc Hs Mt (265) (266) (271) (272) (277) (286) (285) (289) (293) (294) Lv 44 Ru 101.07 76 Os 190.2 61 93 9 VIII U NP 238.03 (237) 27 Co 58.93 45 62 Pm Sm (147) 150.36 Rh 102.91 77 Ir 192.22 109 94 10 VIII 28 46 Pd 106.42 78 63 Eu 151.97 95 11 12 IB IIB Ni Cu Zn 58.69 63.55 65.39 Pu Am (244) (243) 29 30 47 48 Cd 112.41 80 Hg 200.59 5 112 13 IIIA B 10.81 31 Ga 69.72 14 15 IVA VA 49 In 114.82 81 6 12.01 14 16 VIA 64 66 65 67 Gd Tb Dy Ho 157.25 158.93 162.50 164.93 96 97 Cm Bk (247) (247) 17 VIIA 68 69 Er Tm 167.26 168.93 98 99 100 101 Cf Es Fm Md (251) (252) (257) (258) 9 F 19.00 At (210) 18 VIIIA 117 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29. 86 Rn (222) 118 Ts Og (294) 70 71 Yb Lu 173.04 174.97 102 103 No Lr (259) (260)
Step by Step Solution
3.33 Rating (150 Votes )
There are 3 Steps involved in it
We refer to the periodic table to find the group number of the element Chlorine is in Group VIIA17 a... View full answer

Get step-by-step solutions from verified subject matter experts


