Isobutylene (2-methylpropene) can be polymerized by treating it with liquid HF as shown in Fig. P5.53. A
Question:
Isobutylene (2-methylpropene) can be polymerized by treating it with liquid HF as shown in Fig. P5.53. A small amount of tert-butyl fluoride is formed in the reaction. Suggest a curved-arrow mechanism for this process, which is an example of cationic polymerization. (Hint: Carbocations are electrophiles that can react with alkene double bonds.)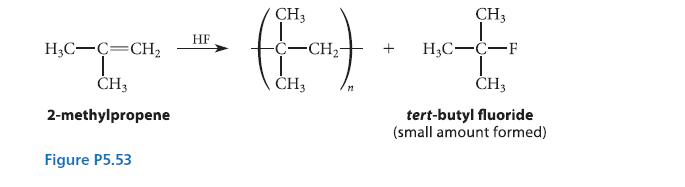
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





