One of the reactions given in Fig. P11.76 is about 2000 times faster in pure water than
Question:
One of the reactions given in Fig. P11.76 is about 2000 times faster in pure water than it is in pure ethanol.
Another is about 20,000 times faster in pure ethanol than it is in pure water. The rate of the third changes very little when the solvent composition is changed from ethanol to water. Which of the reactions is faster in ethanol, which is faster in water, and which has a rate that is solventinvariant?
Explain. The solvent (ethanol, water, or a mixture of the two) in the following equations is indicated by ROH.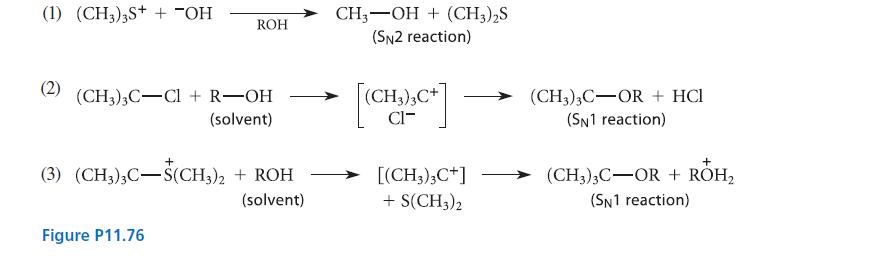
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





