The hydrogenbromine reaction corresponds to the production of HBr(g) from H 2 (g) and Br 2 (g)
Question:
![d[HBr]_ k[H2][Br,]2 тHBr] dt 1+ [Br,]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1526/4/6/3/0085afbfa20b923f1526463004273.jpg)
where k and m are constants. It took 13 years for the correct mechanism of this reaction to be proposed, and this feat was accomplished simultaneously by Christiansen, Herzfeld, and Polyani. The mechanism is as follows:
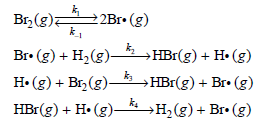
Construct the rate law expression for the hydrogen€“bromine reaction by performing the following steps:
a. Write down the differential rate expression for [HBr].
b. Write down the differential rate expressions for [Br·] and [H·].
c. Because Br· and Hi are reaction intermediates, apply the steady-state approximation to the result of part (b).
d. Add the two equations from part (c) to determine [Br·] in terms of [Br2].
e. Substitute the expression for [Br·] back into the equation for [H·] derived in part (c) and solve for [H·].
f. Substitute the expressions for [Br·] and [H·] determined in part (e) into the differential rate expression for [HBr] to derive the rate law expression for the reaction.
Step by Step Answer:






