Hydrogen abstraction from hydrocarbons by atomic chlorine is a mechanism for Cl · loss in the stratosphere.
Question:
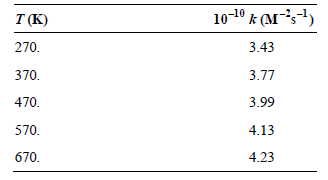
a. Determine the Arrhenius parameters for this reaction.
b. At the tropopause (the boundary between the troposphere and stratosphere located approximately 11 km above the surface of the Earth), the temperature is roughly 220 K. What do you expect the rate constant to be at this temperature?
c. Using the Arrhenius parameters obtained in part (a), determine the Eyring parameters ΔH€¡ and ΔS€¡ for this reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





