In Problem 9.79, the synthesis of methanol from carbon monoxide and hydrogen was described. Further analysis, however,
Question:
In Problem 9.79, the synthesis of methanol from carbon monoxide and hydrogen was described.
Further analysis, however, reveals that three reactions can take place:
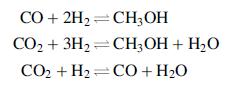
(a) Show that only two of these reactions are independent.
(b) The equilibrium constants for the first and third reactions are
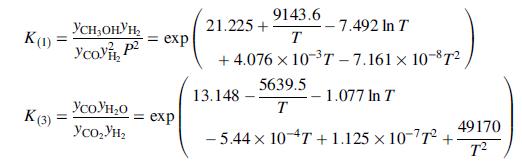
As in Problem 9.79, the feed composition is 5.0 mole% methane, 25.0% CO, 5.0% CO2, and the remainder hydrogen. The temperature and pressure of the equilibrated product stream are 250°C and 7.5MPa. Determine the composition (mole fractions) of the product stream and the percentage conversions of CO and H2.
Problem 9.79
A methanol-synthesis reactor is fed with a gas stream at 220°C consisting of 5.0 mole% methane, 25.0% CO, 5.0% CO2, and the remainder hydrogen. The reactor and feed stream are at 7.5 MPa. The primary reaction occurring in the reactor and its associated equilibrium constant are
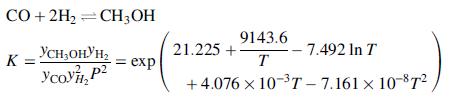
where T is in kelvins. The product stream may be assumed to reach equilibrium at 250°C.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





