Question: For the electron wave function shown in FIGURE Q39.3, at what position or positions is the electron most likely to be found? Least likely to
For the electron wave function shown in FIGURE Q39.3, at what position or positions is the electron most likely to be found? Least likely to be found? Explain.
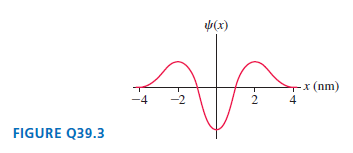
b(x) -x (nm) 4 2 FIGURE Q39.3
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
The probability of finding a particle at position x is ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1442_6054778bf0f1d_701083.pdf
180 KBs PDF File
1442_6054778bf0f1d_701083.docx
120 KBs Word File


