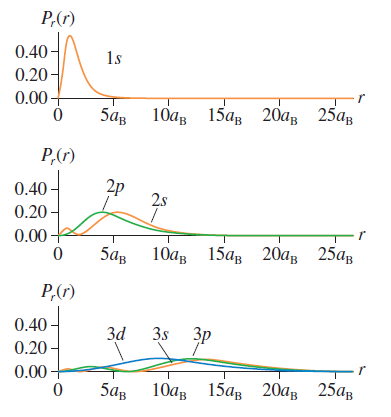
Question: The hydrogen atom 1s wave function is a maximum at r = 0. But the 1s radial probability density, shown in Figure 41.7 , peaks
The hydrogen atom 1s wave function is a maximum at r = 0. But the 1s radial probability density, shown in Figure 41.7, peaks at r = aB and is zero at r = 0. Explain this paradox.
Step by Step Solution
There are 3 Steps involved in it
The radial probability density P r r 4r 2 R nl ris the probability density ... View full answer

Get step-by-step solutions from verified subject matter experts


