Question: 1. {10) The reaction A+2B a C is a second-order in A and zero order in B. The initial concentrations are 0.30M for both A
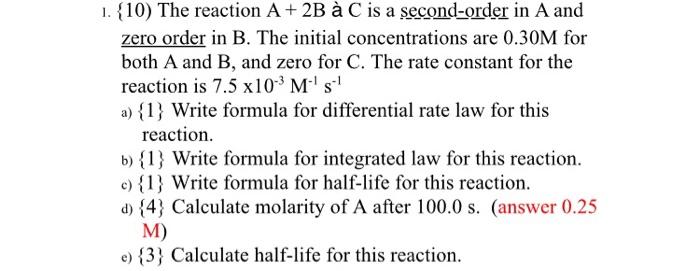
1. {10) The reaction A+2B a C is a second-order in A and zero order in B. The initial concentrations are 0.30M for both A and B, and zero for C. The rate constant for the reaction is 7.5103M1s1 a) {1} Write formula for differential rate law for this reaction. b) {1} Write formula for integrated law for this reaction. c) {1} Write formula for half-life for this reaction. d) {4} Calculate molarity of A after 100.0s. (answer 0.25 M) e) {3} Calculate half-life for this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


