Question: 1. Consider a model polymer undergoing a 2-state folding / unfolding transition at equilibrium. The polymer populates one folded/compact closed state and an unfolded open
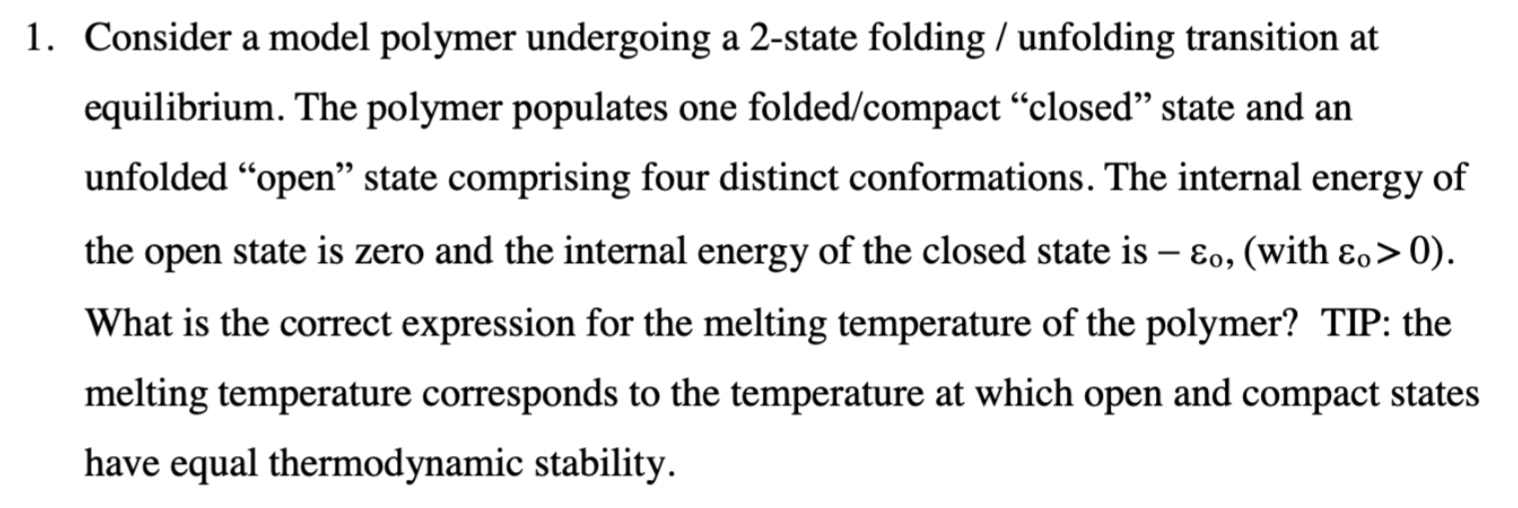
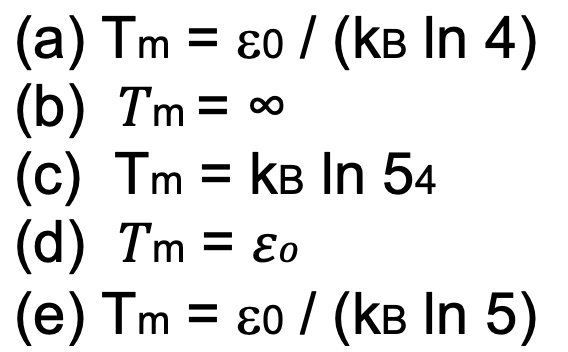
1. Consider a model polymer undergoing a 2-state folding / unfolding transition at equilibrium. The polymer populates one folded/compact closed state and an unfolded "open" state comprising four distinct conformations. The internal energy of the open state is zero and the internal energy of the closed state is 0, (with o > 0). What is the correct expression for the melting temperature of the polymer? TIP: the melting temperature corresponds to the temperature at which open and compact states have equal thermodynamic stability. - (a) Tm = 0 / (kB In 4) o (b) Tm = 20 (c) Tm = kB In 54 (d) T'm = Eo (e) Tm = o / (kb In 5)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


