Question: + 1) For the reaction (CaCO3 + 2HCl CaCl2 + CO2 + H20) between 20 wt% HCl solution (M.W. 36.51b/bmola SG 1.10) and calcite (M.W.100.1
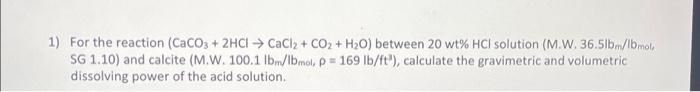
+ 1) For the reaction (CaCO3 + 2HCl CaCl2 + CO2 + H20) between 20 wt% HCl solution (M.W. 36.51b/bmola SG 1.10) and calcite (M.W.100.1 bm/lbmol, P = 169 lb/ft"), calculate the gravimetric and volumetric dissolving power of the acid solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


