Question: 1). For which structure(s) does the electron-bearing carbon atom have a formal charge of zero? 2). For which structure(s) is the net charge zero? 0%
1). For which structure(s) does the electron-bearing carbon atom have a formal charge of zero?
2). For which structure(s) is the net charge zero?
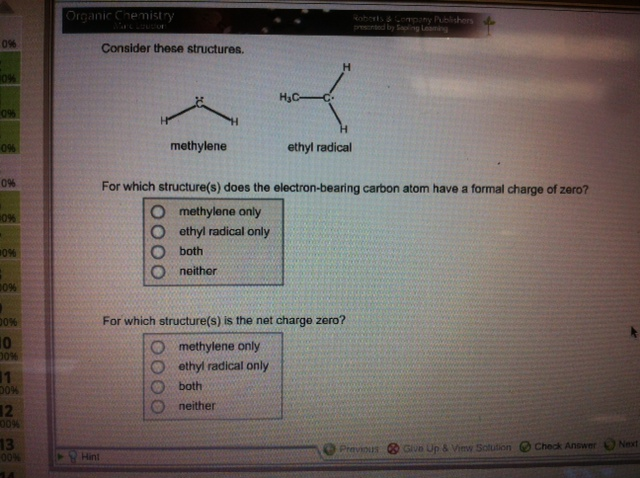
0% 10% 096 0% 0% 0% #0% 00% 00% 10 00% 1 30% 12 00% 13 00% 14 Organic Chemistry Hint Consider these structures. methylene H ethyl radical only O both Oneither HC- ethyl radical For which structure(s) does the electron-bearing carbon atom have a formal charge of zero? O methylene only O Roberts & Company Publishers presented by Sapling Leaming For which structure(s) is the net charge zero? methylene only ethyl radical only both neither Previous Give Up & View Solution Check Answer Next
Step by Step Solution
There are 3 Steps involved in it
Answer and Explanation 1 1 Both 2 Both In both structures there carbon has a formal cha... View full answer

Get step-by-step solutions from verified subject matter experts


